FDA greenlights Boston Scientific’s Novel Drug-Coated Balloon for Coronary In-Stent Restenosis
Legacy MEDSearch
MARCH 1, 2024
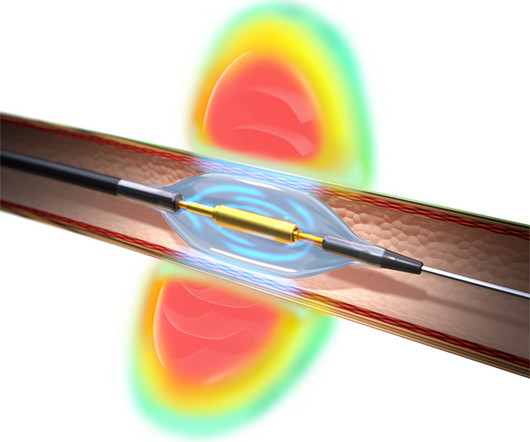
Boston Scientific’s AGENT Drug-Coated Balloon (DCB) has been granted approval by the U.S. Food and Drug Administration (FDA) for treating coronary in-stent restenosis (ISR) in patients with coronary artery disease. physicians the opportunity to treat their patients with this novel device.”





































Let's personalize your content