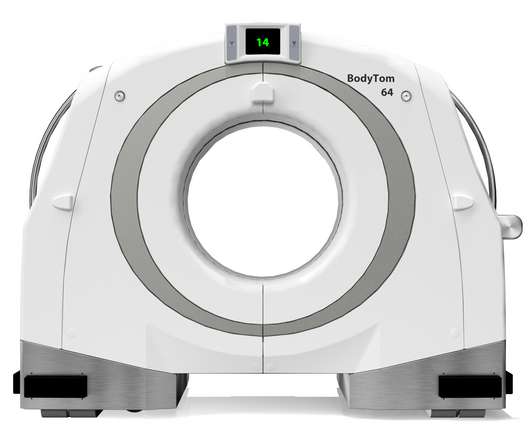
NeuroLogica Announces FDA 510(k) Clearance of BodyTom® 64
Legacy MEDSearch
NOVEMBER 28, 2022
Food and Drug Administration for commercial use in the United States. Trauma/ER: The BodyTom 64’s unique combination of internal lead shielding and battery operation allows any standard trauma bay to be transformed into an advanced CT imaging suite. the healthcare subsidiary of Samsung Electronics Co.,

































Let's personalize your content