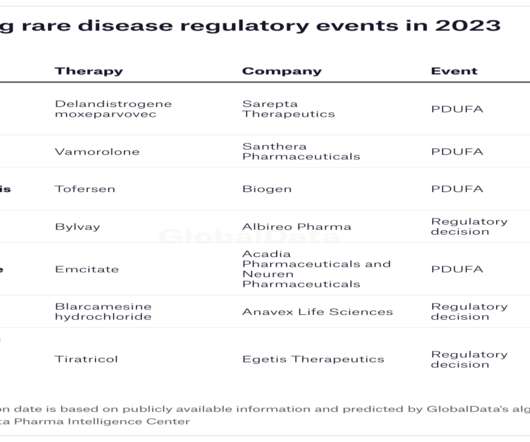
FDA approves Ipsen’s Bylvay for cholestatic pruritus due to ALGS
Pharmaceutical Technology
JUNE 14, 2023
Ipsen has received US Food and Drug Administration (FDA) approval for Bylvay (odevixibat) to treat patients aged 12 months and above with cholestatic pruritus caused by Alagille syndrome (ALGS).























Let's personalize your content