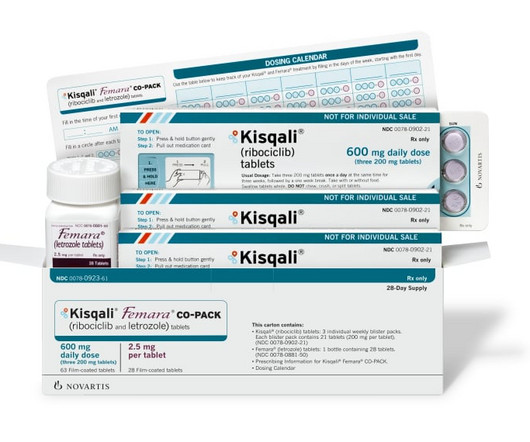
Novartis halts recruitment of Kisqali trials as it adjusts production methods
Fierce Pharma
APRIL 10, 2024
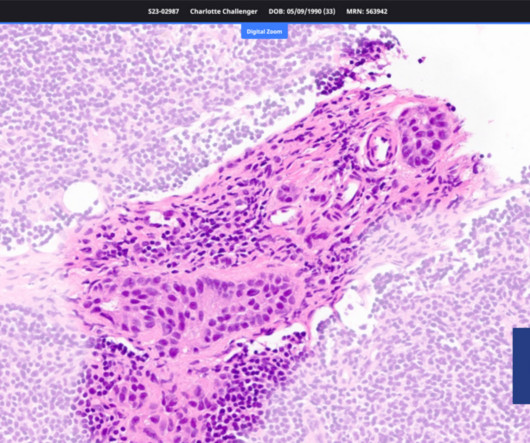
In response to recent FDA guidelines which limit the nitrosamine content of drugs, Novartis has paused the enrollment of early breast cancer (eBC) patients in clinical trials of its blockbuster Kis | In response to recent FDA guidelines which limit the nitrosamine content of drugs, Novartis has paused the enrollment of early breast cancer (eBC) patients (..)


































Let's personalize your content