With 4-year data, Novo bolsters star power of obesity blockbuster Wegovy
Fierce Pharma
MAY 14, 2024
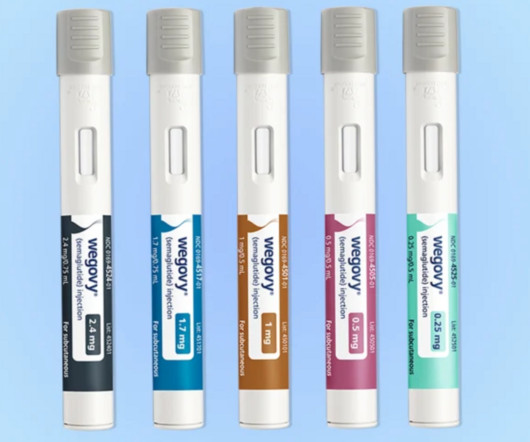
With new four-year data in hand, Novo Nordisk can make a case for its popular obesity med Wegovy to be used as a long-term treatment. | An analysis unveiled at the European Congress on Obesity showed that Wegovy patients lost 10% of their weight, on average, over a 4-year span.




































Let's personalize your content