The ingredients of a successful biopharma collaboration
PharmaVoice
JUNE 13, 2023
Collaboration is important along the entire spectrum and lifecycle of biopharma, and finding an effective partner is harder than it sounds.

PharmaVoice
JUNE 13, 2023
Collaboration is important along the entire spectrum and lifecycle of biopharma, and finding an effective partner is harder than it sounds.

MedReps
JUNE 13, 2023
Communication is important, especially between medical sales reps and sales team leaders. In order for everyone to be on the same page, as well as deal with successes and setbacks accurately, there needs to be open communication. If your medical sales reps are too afraid to talk to you about their issues with the job, such as problems making a certain sale or the inability to find a certain client’s pain point, then they will more than likely not meet their quotas and start looking to find anoth
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

PharmaVoice
JUNE 14, 2023
The big pharma patent cliff is almost here — and could change the face of the industry. Here’s a look at the key stats behind the coming wave of blockbuster losses.

Fierce Pharma
JUNE 12, 2023
Two years after the initial COVID-19 vaccine push swept across the globe, Pfizer’s COVID-19 vaccine partner BioNTech is heading to court in its home country of Germany to defend itself against alle | The drugmaker will defend itself against claims from a German healthcare worker who sued the company for at least 150,000 euros ($161,500). The plaintiff alleges she suffered bodily harm resulting from Pfizer and BioNTech's Comirnaty vaccine.

MedCity News
JUNE 14, 2023
Hospitals’ digital health adoption exploded during the pandemic, leading to many vendor contracts spanning three to five years. As these contracts reach their expiration dates over this year and next, a new report predicts that telemedicine platforms and remote patient monitoring tools face the highest risk of being turned over by hospitals.
Healthcare Success
JUNE 12, 2023
Consumers across the globe have come to rely on search engines for reliable, trustworthy, accurate, and timely healthcare information. This has significantly increased competition among providers, making SEO—particularly local SEO—a crucial part of any digital marketing strategy. Local SEO is beneficial for any size business but critical for multilocation companies.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

Fierce Pharma
JUNE 12, 2023
Bayer recently laid out its ambition | Bayer recently laid out its ambition to achieve $10 billion in sales from its oncology business by 2030 and become a top 10 cancer drug player. To get there, the company is looking outside for a “midsize acquisition,” Bayer’s oncology chief Christine Roth said.

MedCity News
JUNE 13, 2023
Many health systems aren’t employing the right tactics for hiring and retaining nurses, according to a new report. It argued that hospitals would have an easier time hiring and retaining nurses if they focused more on the things workers want most from their employers — such as flexible scheduling and professional development opportunities.

Pharmaceutical Technology
JUNE 15, 2023
Eloxx has revealed its lead candidate ELX-02 improved predicted forced expiratory volume (ppFEV1) in patients with Class 1 cystic fibrosis (CF) in a new analysis of a Phase II trial that missed its efficacy endpoints. Following underwhelming topline results from the Phase II trial (NCT04135495) announced in late 2022, Eloxx recalculated the results using the change in ppFEV1 (a secondary outcome) from day 1 instead of from baseline.

PM360
JUNE 16, 2023
While all patient communities and advocacy groups offer patients, their families, and their caregivers support, advice, and information, rare disease patient groups can be especially tight-knit. With such limited information out there for some rare diseases, these groups are often the only resource for people looking for answers. Furthermore, these patients are typically the ones raising money for research into potential treatments and cures, as well as banding together to try to change governme

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Fierce Pharma
JUNE 16, 2023
In the battle for superiority in the field of next-gen diabetes and obesity treatments, Novo Nordisk holds the lead as the developer of the metabolism-regulating treatment semaglutide. | In the battle for diabetes and obesity superiority, Novo Nordisk holds a head start as the original developer of the metabolism-regulating treatment semaglutide. But Eli Lilly is quickly gaining ground and is primed to become the market leader with its GLP-1 treatment Mounjaro, according to GlobalData.
MedCity News
JUNE 13, 2023
Ipsen drug Bylvay is now FDA approved for treating pruritus, or severe itching, which is a complication of the rare liver disease Alagille syndrome. The oral drug was previously approved for treating pruritus in another rare inherited liver disease called PFIC.

Pharmaceutical Commerce
JUNE 14, 2023
In this latest Harvard Business School Healthcare Alumni Association Q&A, Susan Wilner Golden, DSC, lecturer at the Stanford Graduate School of Business, reveals an untapped $22 trillion global opportunity for all companies and others supporting healthy aging and longer life spans.

PM360
JUNE 16, 2023
According to the National Organization for Rare Disorders (NORD) , there are over 7,000 known rare diseases, with 90% having no effective treatment method or cure. 1 When an individual is diagnosed with a rare disease, they often experience fear, uncertainty, and anxiety. Obtaining a diagnosis is only the first step, leaving many without the direction or resources to find the additional medical care they need.

Advertiser: ZoomInfo
Marketing technology is essential for B2B marketers to stay competitive in a rapidly changing digital landscape — and with 53% of marketers experiencing legacy technology issues and limitations, they’re researching innovations to expand and refine their technology stacks. To help practitioners keep up with the rapidly evolving martech landscape, this special report will discuss: How practitioners are integrating technologies and systems to encourage information-sharing between departments and pr
Fierce Pharma
JUNE 16, 2023
Merck’s cancer star Keytruda could be on its way to an updated label in HER2-positive stomach cancer after showing it can stave off tumor progression in a combination study. | After scoring accelerated approval to treat HER2-positive stomach cancer in 2021, the drug has now shown it can stave off tumor progression in patients with PD-L1 positive tumors.

MedCity News
JUNE 16, 2023
To increase your return on technology investment, start with an audit of existing technology. Where is it falling short? Do problems stem from lack of functionality or troubles with adoption? What do all users think?
Pharmaceutical Technology
JUNE 13, 2023
Beacon Therapeutics has kickstarted its entry into the gene therapy field with a $120m Series A financing. The British investment trust Syncona Limited launched the new ophthalmic gene therapy company by combining Applied Genetic Technologies Corporation’s (AGTC’s) late-stage X-linked retinitis pigmentosa (XLRP) programme with two proprietary preclinical programmes.
Legacy MEDSearch
JUNE 15, 2023
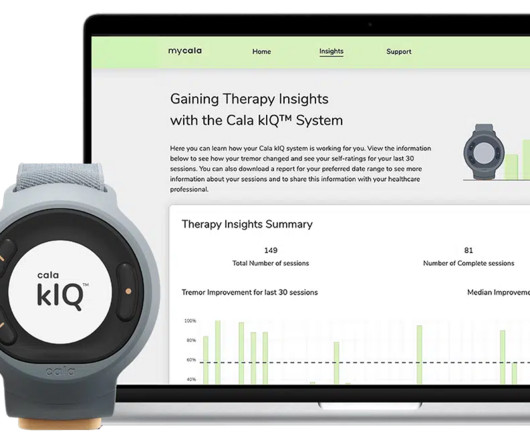
Cala , the bioelectronic medicine leader setting a new standard of care for chronic disease, today announced the commercial launch of its next generation system: the Cala kIQ System, the first and only FDA-cleared wearable device that delivers effective therapy for action hand tremor relief in people with essential tremor and Parkinson’s disease. The Cala kIQ System delivers Cala TAPS (Transcutaneous Afferent Patterned Stimulation) therapy, which is validated by large clinical studies and real-w

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Fierce Pharma
JUNE 14, 2023
Sanofi is jumping on the artificial intelligence boat with a new app and a pledge to become “the first pharma company powered by artificial intelligence at scale.” | Sanofi's new app, plai, provides teams with a “360° view" to aid decision-making. The platform is one step in the company's digital transformation and goal to become the "first pharma powered by AI at scale.

MedCity News
JUNE 16, 2023
The consensus is 2022 was a challenging year for digital health companies. Venture capital and other startup investment in the U.S. digital health sector plummeted to $15 billion from more than $29 billion in 2021, according to Rock Health. Market analysts CB Insights similarly tracked private investment last year in U.S. digital health at $17.7 billion in 2022 — down 56% from $40.2 billion in 2021.
Pharmaceutical Technology
JUNE 13, 2023
The US Food and Drug Administration (FDA) has accepted AstraZeneca’s new drug application (NDA) for the combination of capivasertib and FASLODEX (fulvestrant), and granted it priority review. The combination therapy is intended to treat hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative locally advanced or metastatic breast cancer in adult patients, after recurrence or progression on or after an endocrine-based regimen.
European Pharmaceutical Review
JUNE 13, 2023
A new therapeutic radionuclide facility, the world’s largest production site of lutetium-177, has opened in Germany. ITM Isotope Technologies Munich SE ( ITM )’s manufacturing plant in Neufahrn near Munich will produce the innovative medical isotope for targeted cancer therapies. “Radiopharmaceuticals are an essential new class of anti-cancer drugs that have the potential to improve therapy outcomes and quality of life for many patients.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Fierce Pharma
JUNE 16, 2023
Less than a month after AbbVie and Genmab won FDA approval for Epkinly, Roche has crossed the finish line with its bispecific answer to large B-cell lymphoma. | Less than a month after AbbVie and Genmab won FDA approval for Epkinly, Roche has crossed the finish line with its bispecific answer to large B-cell lymphoma, though with a narrower label.

MedCity News
JUNE 16, 2023
The FDA approved Roche’s Columvi as a third-line treatment for a type of blood cancer called diffuse large B-cell lymphoma. This new Roche drug will compete against Epkinly, AbbVie’s recently approved DLBCL drug.
Pharmaceutical Technology
JUNE 14, 2023
The US Food and Drug Administration (FDA) has granted fast track designation for CellCentric’s inobrodib (CCS1477) to treat relapsed or refractory multiple myeloma patients. Inobrodib is an oral first-in-class cancer drug, indicated for patients who have previously received four or more lines of therapy, including an anti-CD38 monoclonal antibody, an immunomodulatory agent and a proteasome inhibitor.

European Pharmaceutical Review
JUNE 11, 2023
The US Food and Drug Administration (FDA) has approved commercial production at Bristol Myers Squibb’s newest cell therapy manufacturing facility in Devens, Massachusetts. The 244,000 square foot facility in Devens is BMS’ third commercial cell therapy facility in the US. It is located on the company’s existing Devens site, which has been developing, producing, and testing clinical and commercial medicines for over a decade.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

Fierce Pharma
JUNE 14, 2023
An FTC lawsuit aimed at blocking Amgen’s $27.8 billion buyout o | An FTC lawsuit aimed at blocking Amgen’s $27.8 billion buyout of Horizon Therapeutics has raised concerns that the United States’ antitrust watchdog is tightening the screws on major M&A moves in the biopharma industry. A month later, according to an SEC filing, Pfizer has withdrawn its notification for its proposed $43 billion acquisition of cancer drug specialist Seagen and will submit another later in the day.

MedCity News
JUNE 11, 2023
The Making Care Primary Model will be tested by the Center for Medicare and Medicaid Innovation from July 1, 2024, to December 31, 2034, in Colorado, Massachusetts, Minnesota, New Jersey, New Mexico, New York, North Carolina and Washington.

Pharmaceutical Technology
JUNE 12, 2023
Lotus Pharmaceuticals and Teraju Pharma have entered a strategic partnership to market Lotus’s products in a range of therapeutic areas in Malaysia. Teraju Pharma will be responsible for marketing and promoting the products using its relationships and infrastructure. The company is a specialised pharmaceutical wholesaler and holds a licence, issued by the country’s ministry of health, to import non-registered pharmaceutical products, orphan drugs and other life-saving medicines.

Spotio
JUNE 13, 2023
At the end of every month, sales managers may be asking themselves a single question: Why is my sales team falling short of their selling quota? Sales metrics might answer that question, but too often, sales managers look at metrics that are outside of their control. A Harvard Business Review study found that of 306 metrics sales managers track, only 17% are sales activities.

Advertiser: ZoomInfo
In times of economic uncertainty, account-based strategies are essential. According to several business analysts and practitioners, ABM is a necessity for creating more predictable revenue. Research shows that nearly three-quarters of marketers (74%) already have the resources needed to build successful ABM programs.
Let's personalize your content