With FDA rejection to treat hives disorder, Sanofi and Regeneron's Dupixent suffers rare setback
Fierce Pharma
OCTOBER 23, 2023
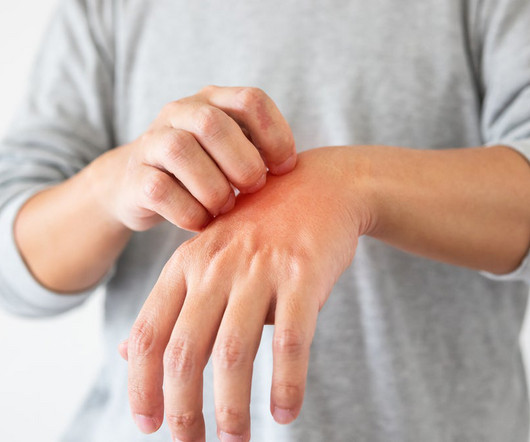
Regeneron and Sanofi are anxious to bring Dupixent as an answer for chronic spontaneous urticaria (CSU), a severe inflammatory condition that causes hives and deep swelling on or under the skin.





































Let's personalize your content