As pharma fights IRA, Senate moves forward basket of bills aimed at lower drug prices
Fierce Pharma
MAY 12, 2023
As pharma fights IRA, Senate moves forward basket of bills aimed at lower drug prices aliu Fri, 05/12/2023 - 10:48

Fierce Pharma
MAY 12, 2023
As pharma fights IRA, Senate moves forward basket of bills aimed at lower drug prices aliu Fri, 05/12/2023 - 10:48

MedCity News
MAY 12, 2023
Although conversations around other LGBTQ+ issues are prevalent in mainstream culture, more work needs to be done on understanding our screening, treatment, and survivorship.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
MAY 12, 2023
Pfizer CEO Bourla slams Medicare IRA measure as 'negotiation with a gun to your head' kdunleavy Fri, 05/12/2023 - 10:32
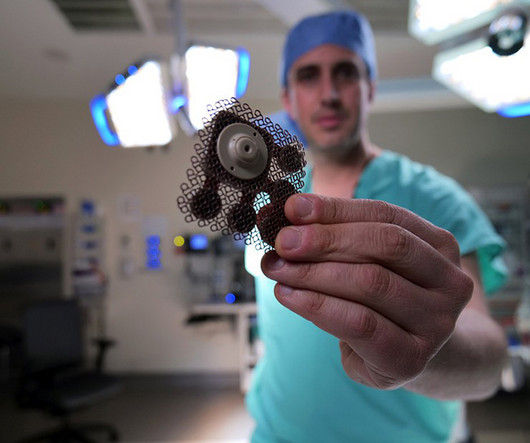
Medgadget
MAY 12, 2023
Researchers at Northwestern University have trailed an implanted ultrasound device in patients, which is used in combination with microbubbles to transiently open pores in the blood brain barrier, allowing chemo drugs to enter. We have reported on this technique before as a lab-based concept (see flashbacks below), but this is the first time that it has actually been trialed in human patients, in this case patients with glioblastoma, a difficult to treat brain cancer.

Fierce Pharma
MAY 12, 2023
Telehealth is here to stay, survey shows. How should the industry adapt?

MedCity News
MAY 12, 2023
To truly solve provider shortages—and get providers practicing sooner—health systems and plans need to automate licensing, enrollment, and credentialing, so they can scale their provider networks and meet increasing patient demand.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.
European Pharmaceutical Review
MAY 12, 2023
Swedish Orphan Biovitrum AB (Sobi ® ) has agreed to acquire CTI BioPharma for $1.7 billion, with the intention to advance an oral kinase inhibitor treatment for the rare disease myelofibrosis. Innovative therapies for rare diseases The acquisition will help to drive the growth of CTI’s lead product, kinase inhibitor VONJO ® (pacritinib) “in treating myeloproliferative disease,” stated Dr Adam Craig, President, Chief Executive Officer and Interim Chief Medical Officer of CTI BioPharma

Fierce Pharma
MAY 12, 2023
After costly setback, Astellas' menopause drug crosses FDA finish line zbecker Fri, 05/12/2023 - 16:21
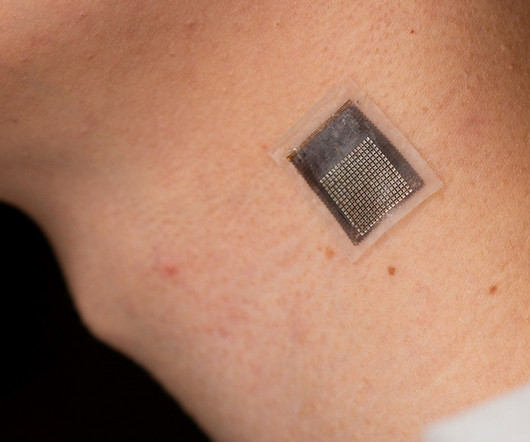
Medgadget
MAY 12, 2023
Researchers at the University of California San Diego have developed a wearable ultrasound patch that is intended to provide information on the stiffness of underlying tissues as deep as 4 cm below the surface of the skin. The patch consists of a flexible 16 x 16 ultrasonic array with a silver-epoxy composite backing layer that is designed to absorb excessive vibrations.

Fierce Pharma
MAY 12, 2023
Takeda braces for impact as Vyvanse edges toward the patent cliff fkansteiner Fri, 05/12/2023 - 10:55

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Pharmaceutical Technology
MAY 12, 2023
The Technical University of Denmark (DTU) has announced that an international scientific team has developed a CRISPR-based drug candidate that targets E coli directly and leaves the microbiome intact. Blood cancer patients with E coli infection are at risk of bacteria infecting the bloodstream. An E coli infection can be fatal in these cases, with a 15-20% mortality rate.

PharmExec
MAY 12, 2023
Stefan Merlo, Vice President, Commercial Development, CSL Seqirus discusses how COVID-19 has evolved the discussion of vaccines and the long-term concerns stemming from flu vaccine fatigue.

Pharmaceutical Technology
MAY 12, 2023
Takeda has reported a 12.8% increase in its reported revenue to $29.96bn (Y4,027bn) during the fiscal year 2022 (FY2022) compared to that reported in FY2021. At a constant exchange rate (CER), the company’s core revenue grew by 3.5% compared to the previous year. In the FY2022 ending 31 March 2023, the company also reported a 6.4% rise in its operating profit to $3.65bn (Y490.5bn) compared to 2021.

MedCity News
MAY 12, 2023
Carbon went out of Anthem Blue Cross’ network March 17 after not coming to an agreement on reimbursement. The company took the dispute public in an April 29 blog post declaring the rate Anthem pays its providers as not a livable wage. These kinds of disputes between payers and providers happen frequently, though they’re usually not as public, one expert said.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Pharmaceutical Technology
MAY 12, 2023
Editas Medicine will release new efficacy and safety results of its gene therapy EDIT-301 in severe sickle disease as part of an oral presentation at the European Hematology Association’s (EHA) Hybrid Congress. The EHA Hybrid Congress will take place on June 8-11 in Frankfurt, Germany. Last month, the US Food and Drug Administration (FDA) granted the treatment an orphan drug designation for sickle cell disease.

MedCity News
MAY 12, 2023
Employers should be looking at obesity as a chronic condition and focus on weight management solutions over weight loss solutions, said Parin Chikani, medical account director for managed markets at Novo Nordisk. He made these comments Tuesday at the Midwest Business Group on Health conference in Chicago.
European Pharmaceutical Review
MAY 12, 2023
A BioNTech-led Phase I trial demonstrated preliminary evidence that adjuvant autogene cevumeran, a personalised mRNA neoantigen vaccine, in combination with atezolizumab (Genentech) and mFOLFIRINOX can induce substantial T cell activity in pancreatic ductal adenocarcinoma (PDAC). In a paper published in Nature , researchers hypothesised it may correlate with delayed recurrence and elimination of micrometastases in PDAC.

MedCity News
MAY 12, 2023
While we always say that patients are the center of what we do, fragmentation and shifting priorities can make true patient-led care difficult to achieve.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

Copyright Clearance Center
MAY 12, 2023
The post 2023 Pulitzers To Kingsolver, Diaz, Others appeared first on Copyright Clearance Center.
MedCity News
MAY 12, 2023
The antibody drug, nirsevimab, is designed with a long half-life intended to protect infants from respiratory syncytial virus infection through the entire season. The drug, co-developed by AstraZeneca and Sanofi, is under FDA review.
Pharmaceutical Technology
MAY 12, 2023
On 8 May 2023, China-based Bliss Biopharmaceutical (BlissBio) announced a clinical trial collaboration with Eisai to develop BB-1701, an antibody-drug conjugate (ADC) for multiple cancer types. The agent is a human epidermal growth factor receptor 2 (HER2)-targeting ADC that utilises Eisai’s chemotherapy Halaven (eribulin) as its payload. Under the agreement, BlissBio will receive an undisclosed sum in upfront and milestone payments, and the companies will codevelop BB-1701 through an option per

PharmaTimes
MAY 12, 2023
Confederation responds to generally positive statistics, including cancer backlog drop

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Spotio
MAY 12, 2023
A sales engagement platform is one of the most powerful tools in your selling arsenal. However, it’s also something that many teams can overlook, or misunderstand. A sales engagement platform isn’t a feature of your CRM, or a collaboration tool. These services are dedicated solutions, created to enhance your workflow across all sales tools, from CRM technology, to your inbox, phone, content management system, and so much more.

PharmaTimes
MAY 12, 2023
The first unit of its kind in England will identify biomarkers and diagnose cancer much faster

PharmExec
MAY 12, 2023
New federal law introduces significant changes in the design of pharmacy benefit plans with aims to level the playing field between PBMs and their employee healthcare benefit plan clients.

Pharmaceutical Commerce
MAY 12, 2023
New federal law introduces significant changes in the design of pharmacy benefit plans with aims to level the playing field between PBMs and their employee healthcare benefit plan clients.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

Cesare Ferrari
MAY 12, 2023
Welcome back! Still, on MedTech over-engineering, I think this sentence from “ The Elements of Style ” is a great guideline for over-writing and can be transferred to over-engineering: “A sentence should contain no unnecessary words, a paragraph no unnecessary sentences, for the same reason that a drawing should have no unnecessary lines and a machine no unnecessary parts.” After analyzing the major causes of over-engineering in MedTech, now is the time to understand the

Pharmaceutical Technology
MAY 12, 2023
BioNTech has ended its research collaboration with Matinas after its oral mRNA vaccine failed to demonstrate preclinical activity. Matinas announced the news in a May 10 statement. The in vivo study, conducted in mice, involved a phosphatidylserine-containing nano-formulation of mRNA supplied by BioNTech, distinct from traditional lipid nanocrystals (LNCs).

Pharma Leaders
MAY 12, 2023
Swiss company SGS has purchased a majority stake in Nutrasource Pharmaceutical and Nutraceutical Services as well as its subsidiaries (Nutrasource). The company will initially buy a 60% interest in Nutrasource while having the right to pick the remaining 40% holding in 2026. Financial terms of the transaction were not shared. Founded in 2001 and headquartered in Guelph, Ontario, Canada, Nutrasource is a global contract research organisation (CRO) in the pharmaceutical and nutraceutical businesse

Pharmaceutical Technology
MAY 12, 2023
Speciality pharmaceutical company SERB Pharmaceuticals has acquired exclusive US rights for bentracimab from SFJ Pharmaceuticals. SFJ Pharmaceuticals will lead the ongoing clinical trial of bentracimab. In partnership with SERB Pharmaceuticals, the company will also file a biologics licence application (BLA) to the US Food and Drug Administration (FDA) later this year.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content