New MD Anderson Research Uncovers Drug Combo That Could Eliminate Pancreatic Cancer Tumors
MedCity News
AUGUST 27, 2023
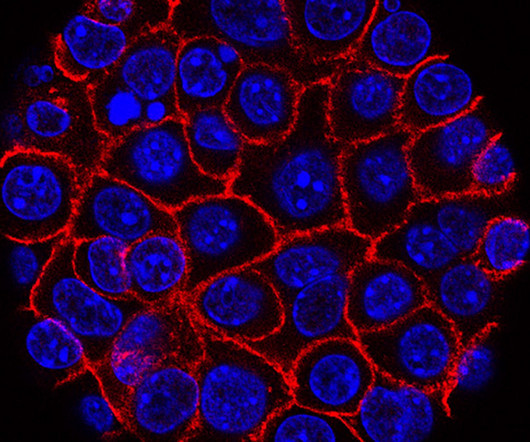
Researchers at the University of Texas MD Anderson Cancer Center published two studies this week on a new approach that could improve treatment for patients with pancreatic cancer. The preclinical studies showed that combining immunotherapy with a KRAS inhibitor can lead to long-lasting tumor elimination.

































Let's personalize your content