Refillable Device for Drug Delivery Past the Blood-Brain Barrier: Interview with Mike Maglin, CEO at CraniUS
Medgadget
SEPTEMBER 29, 2023
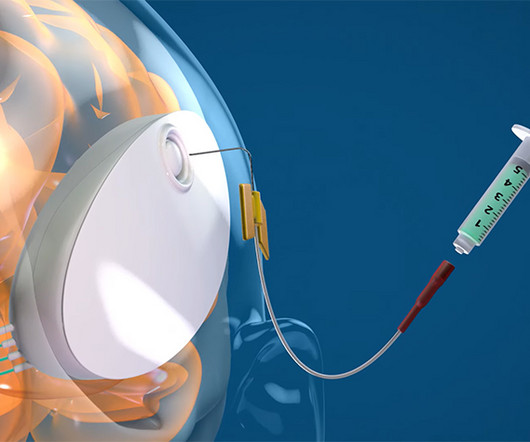
This layer of specialized endothelium significantly restricts which drug molecules can enter the brain, normally greatly limiting treatment options for patients with brain-based disease. CraniUS , a medtech company based in Baltimore, has developed the NeuroPASS drug delivery system.






















Let's personalize your content