Upsized Kyverna IPO Gets $319M to Bring CAR T-Therapy to Autoimmune Diseases
MedCity News
FEBRUARY 8, 2024
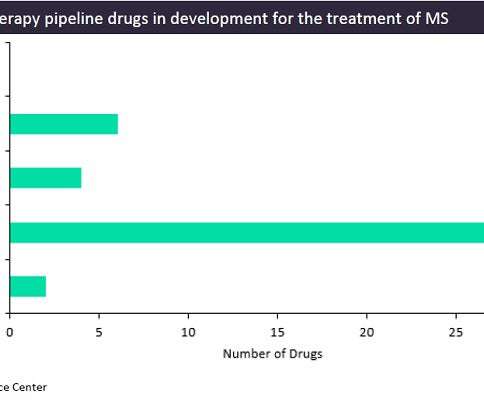
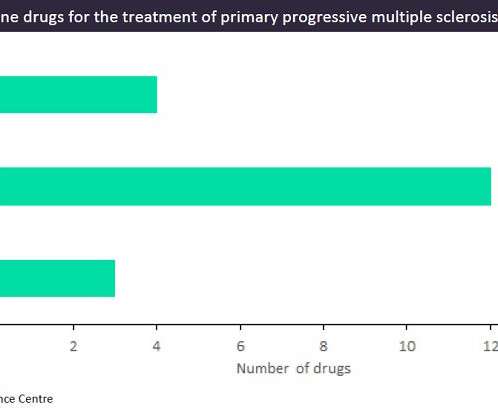
The biotech plans to deploy its IPO cash across clinical trials in lupus nephritis, multiple sclerosis, systemic sclerosis, and myasthenia gravis. Cell therapies were first validated in cancer, but Kyverna Therapeutics aims to show they’re safe enough for autoimmune diseases.





























Let's personalize your content