Research predicts 2029 small molecule oncology market leaders
European Pharmaceutical Review
OCTOBER 12, 2023
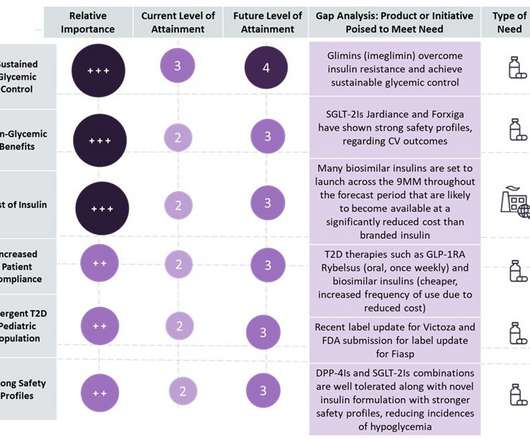
Strong demand for small molecules as treatment for non-small cell lung cancer (NSCLC) in the eight major markets means that the small molecule treatment market for the disease is expected to reach over $15 billion by 2029. Roche is expected to secure second position in the market, due to projected sales of over $2.5 percent by 2029.














Let's personalize your content