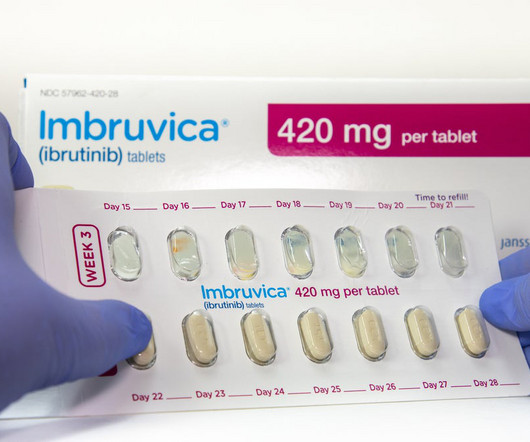
NICE recommends AbbVie’s/J&J’s Imbruvica for combo use in untreated CLL
Pharmaceutical Technology
APRIL 24, 2023
The combination therapy also caused fewer side effects, with heart problems and hypertension as the most common ones, based on the final draft guidance. Brukinsa was approved by the US Food and Drug Administration (FDA) in CLL in January 2023. billion in 2029, while Calquence is expected to make $5.31 billion in 2029.








Let's personalize your content