NICE recommends AbbVie’s/J&J’s Imbruvica for combo use in untreated CLL
Pharmaceutical Technology
APRIL 24, 2023
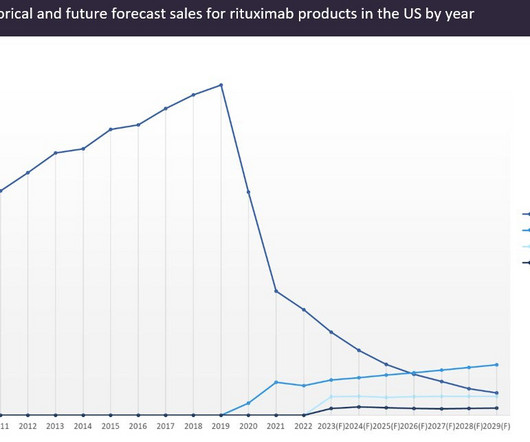
While Imbruvica maintained a previously strong position on the market, competition has chipped away at the inhibitor’s market share. Brukinsa was approved by the US Food and Drug Administration (FDA) in CLL in January 2023. billion in 2029, while Calquence is expected to make $5.31 billion in 2029.












Let's personalize your content