Elevating pharmaceutical manufacturing processes with real-time insights
European Pharmaceutical Review
DECEMBER 4, 2023
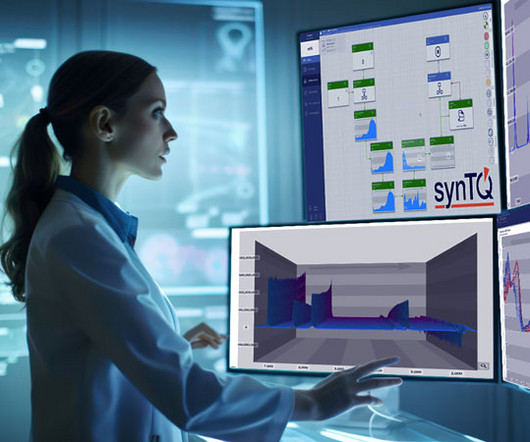
However, the knowledge generated using a PAT framework can help to seamlessly transfer these insights to pilot testing and manufacturing operations. The result is efficient, cost-effective and high-quality (bio)pharmaceutical production. In April 2022 both companies were acquired by Bruker.














Let's personalize your content