Clinical trials authorised for 3D-printed ulcerative colitis drug
European Pharmaceutical Review
NOVEMBER 22, 2022
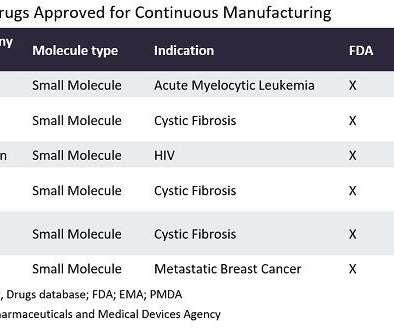
a China-based pharma company has announced it has received clearance for its Investigational New Drug (IND) application from the US Food and Drug Administration (FDA) to initiate clinical studies of T21, a 3D-printed medicine that can target specific segments in the colon to more safely deliver oral ulcerative colitis (UC) drugs.

































Let's personalize your content