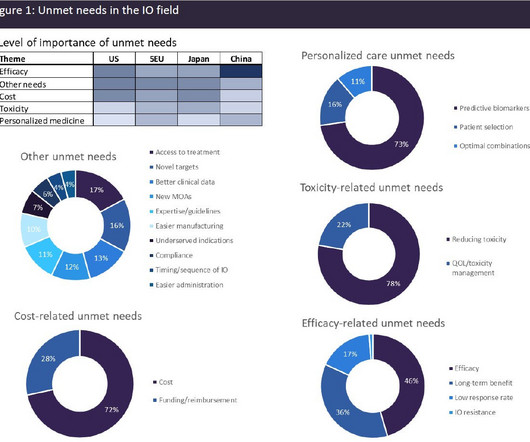
A broad range of unmet needs remains in the immuno-oncology space
Pharmaceutical Technology
MAY 2, 2023
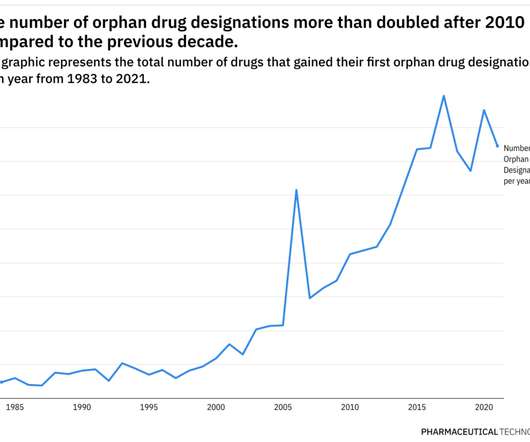
Despite the significant advances that have been made in the IO field, a huge level of unmet need remains. High-prescribing physicians surveyed by GlobalData across eight major markets (8MM: US, France, Germany, Italy, Spain, UK, Japan, and China) identified a broad range of unmet needs in the IO space (see Figure 1).










Let's personalize your content