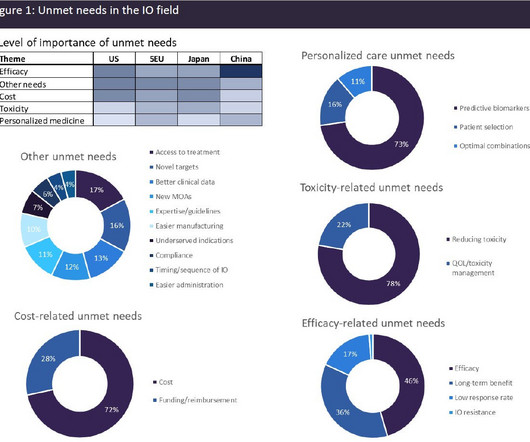
A broad range of unmet needs remains in the immuno-oncology space
Pharmaceutical Technology
MAY 2, 2023
The most widely utilised IO class is immune checkpoint inhibitors (ICIs), which work by blocking the inhibitory interactions between tumour cells and T-cells, facilitating an anti-tumour immune response. There are more than 20 marketed ICIs with approvals across a very wide spectrum of solid tumour indications.












Let's personalize your content