Bacteria Tag Team Tumors with T Cells
Medgadget
OCTOBER 31, 2023
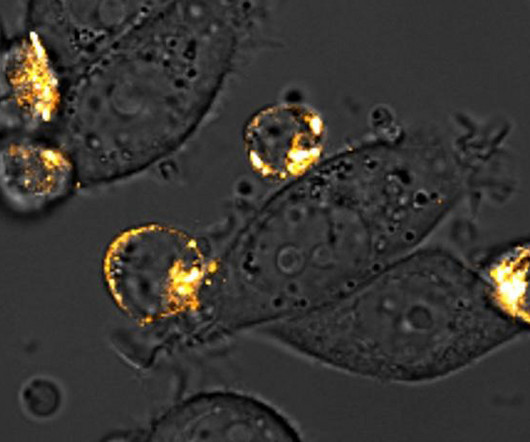
A team at Columbia University School of Engineering and Applied Science has developed a technique to enhance chimeric antigen receptor (CAR) T cell therapy in solid tumors. coli bacteria, that naturally tend to accumulate in the immune privileged core of solid tumors. The technique involves engineering E.











Let's personalize your content