Disc wins orphan drug tag for rare blood cancer
Pharmaceutical Technology
FEBRUARY 12, 2024
The humanised monoclonal antibody DISC-3405 is under investigation in a Phase I clinical trial, with data expected this year.

 tag blood-cancer
tag blood-cancer 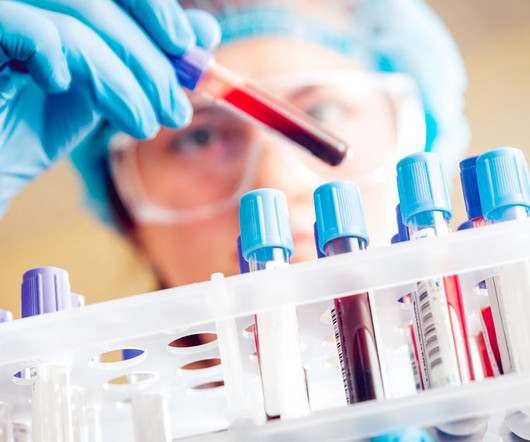
Pharmaceutical Technology
FEBRUARY 12, 2024
The humanised monoclonal antibody DISC-3405 is under investigation in a Phase I clinical trial, with data expected this year.
Medgadget
OCTOBER 31, 2023
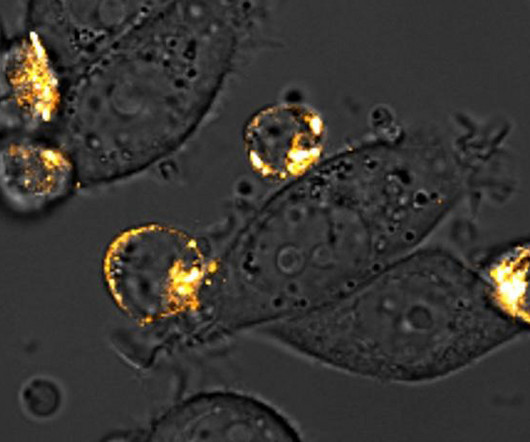
The approach could enhance CAR T cell therapy in solid tumors, which hasn’t worked as well as CAR T cell therapy for blood-borne cancers to date. While this approach has worked reasonably well in blood-borne cancers, such as leukemia, it has proven more difficult to target solid tumors.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
pharmaphorum
DECEMBER 23, 2022
There’s been huge progress in treatments for blood cancer in recent years – but drawbacks of expensive CAR-T cell and injected antibody therapies have led drug developers to look at novel oral therapies as patient-friendly alternatives. These are particularly important in the progression of certain blood cancers.
pharmaphorum
AUGUST 18, 2022
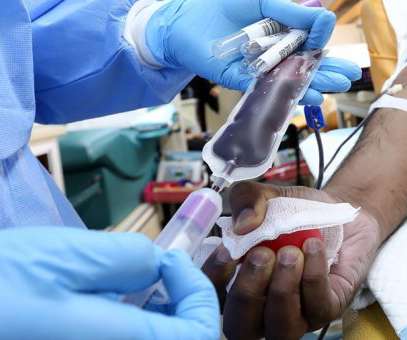
bluebird bio’s Zynteglo has become the first cell-based gene therapy to be approved in the US, getting the nod from the FDA as a treatment for patients with beta thalassaemia who require regular blood transfusions. An FDA advisory committee recommended approval of Zynteglo on the strength of that data in June.

pharmaphorum
AUGUST 25, 2022
Tecvayli (teclistamab) has been approved by the European Commission for adults with the blood cancer whose disease has progressed have after at least three prior therapies, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody, said J&J.

pharmaphorum
DECEMBER 12, 2022
. “This is exciting news because existing treatments fail to improve platelet counts for as many as 30% of adults with chronic ITP,” commented said Catherine Broome of Georgetown Lombardi Cancer Centre in Washington DC, the trial’s lead investigator.

pharmaphorum
JULY 25, 2022
The EMA’s human medicines committee has recommended approval of Johnson & Johnson’s Tecvayli as a fourth-line therapy for multiple myeloma , joining a growing group of BCMA-targeted therapies for the blood cancer.
Let's personalize your content