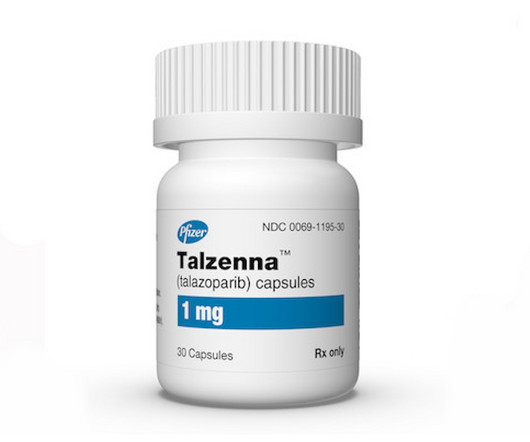
After Pfizer offers discount, NICE gives thumbs up to breast cancer drug Talzenna
Fierce Pharma
JANUARY 18, 2024
Six months after England’s National Institute for Health and Care Excellence (NICE) spurned Pfizer’s breast cancer treatment Talzenna (talazoparib) because it was not cost effective, the agency has | Six months after England’s National Institute for Health and Care Excellence spurned Pfizer’s breast cancer treatment Talzenna because it was not cost (..)







































Let's personalize your content