Ocugen’s retinal therapy gains FDA orphan drug status
Pharmaceutical Technology
APRIL 28, 2023
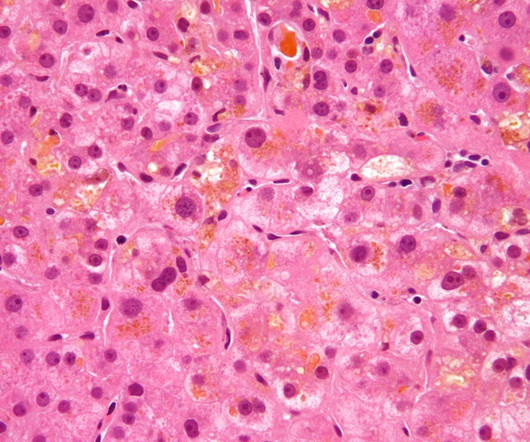
Ocugen has received orphan drug designation from the US Food and Drug Administration for its OCU410ST (AAV5-hRORA) to treat ABCA4 – linked retinopathies. The gene mediates the transport and removal of all-trans-retinal aldehyde, a byproduct of the retinoid cycle of vision, from photoreceptor cells.

















Let's personalize your content