US FDA grants approval for Orion-Bayer’s Nubeqa combo for prostate cancer
Pharmaceutical Technology
AUGUST 8, 2022
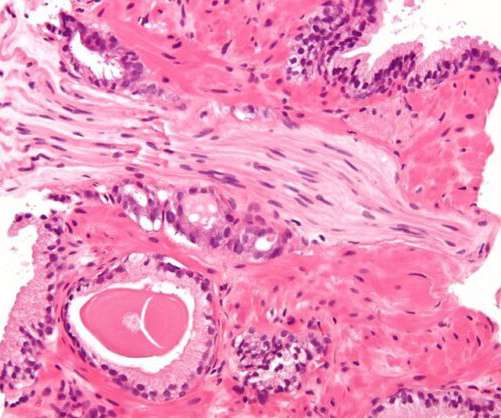
The US Food and Drug Administration (FDA) has granted approval for a supplemental New Drug Application (sNDA) of Orion and its partner Bayer ’s Nubeqa (darolutamide) plus docetaxel to treat metastatic hormone-sensitive prostate cancer (mHSPC) patients. Overall survival was the trial’s primary endpoint.































Let's personalize your content