US FDA grants orphan drug status to IN8bio’s INB-400 and INB-410
Pharmaceutical Technology
APRIL 26, 2023
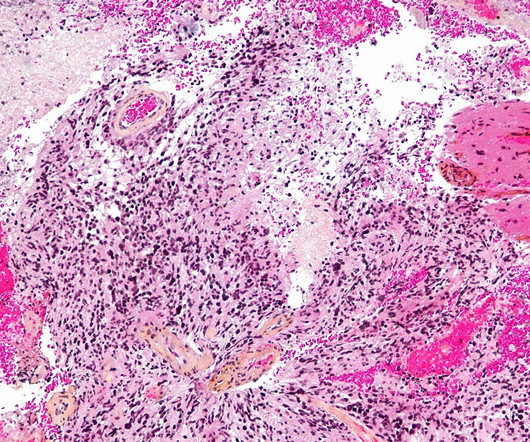
The US Food and Drug Administration (FDA) has granted orphan drug designation to IN8bio’s INB-400 and INB-410 to treat a range of malignant gliomas, including newly diagnosed glioblastoma multiforme (GBM). We eagerly anticipate enrolling our first Phase II patients for INB-400 later this year.”




















Let's personalize your content