AI-Designed Custom Knee Implants
Medgadget
OCTOBER 13, 2022
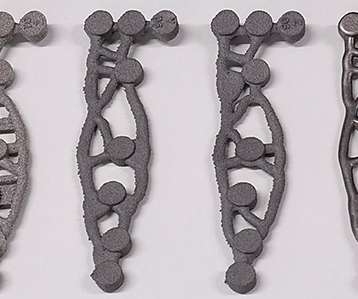
Scientists at the University of Birmingham in the UK have trialed Generative Design, a design approach that relies on machine learning and artificial intelligence, to create patient-specific knee implants. They then used an electron beam powder bed fusion to manufacture their designs.




















Let's personalize your content