Health Canada approves Jazz’s cannabis derived seizure therapy
Pharmaceutical Technology
NOVEMBER 24, 2023
Epidiolex has been approved as an adjunct therapy for seizures associated with three rare forms of epilepsy in patients aged two and older.

Pharmaceutical Technology
NOVEMBER 24, 2023
Epidiolex has been approved as an adjunct therapy for seizures associated with three rare forms of epilepsy in patients aged two and older.
European Pharmaceutical Review
NOVEMBER 24, 2023
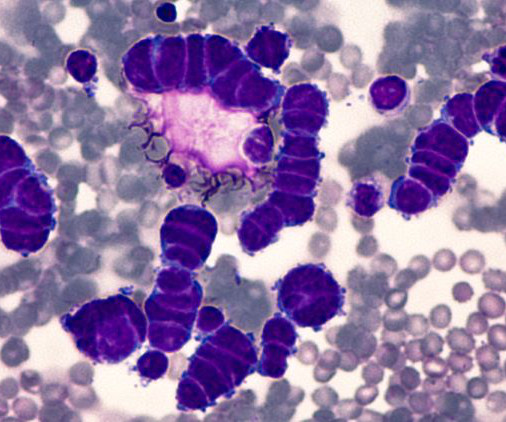
Amgen’s new immunotherapeutic agent Tarlatamab has been shown in a Phase II trial to provide sustained anti-tumour activity in 40 percent of the small cell lung cancer patients. The international DeLLphi-301study was investigated as a new anti-cancer treatment option for patients previously considered to be beyond treatment. Results from the trial were published in the New England Journal of Medicine.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
NOVEMBER 24, 2023
Bayer announced the launch of a Berlin, Germany-based manufacturing facility to meet future demands from the US, China and Europe.

European Pharmaceutical Review
NOVEMBER 24, 2023
Novo Nordisk is investing over €2.1 billion (16 billion Danish kroner) to expand its production site in Chartres, France, supporting manufacture of its products for serious chronic diseases. Novo Nordisk’s multi-billion facility expansion This financial commitment will greatly strengthen manufacturing capacity, more than doubling the site’s footprint.

PharmaTimes
NOVEMBER 24, 2023
Around 3,200 new cases of cervical cancer are diagnosed in the UK every year - News - PharmaTimes
European Pharmaceutical Review
NOVEMBER 24, 2023
The European Commission has granted a label expansion of the combination treatment KAFTRIO ® (ivacaftor/tezacaftor/elexacaftor) with ivacaftor, for children aged two to five years old with cystic fibrosis. The treatment from Vertex Pharmaceuticals is indicated for individuals who have at least one F508del mutation in the cystic fibrosis transmembrane conductance regulator ( CYSTIC FIBROSISTR ) gene.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

PharmaTech
NOVEMBER 24, 2023
The largest biotech hub is set to be built in Lithuania by Northway Group.

pharmaphorum
NOVEMBER 24, 2023
International Health Partners' Christmas campaign: Working with pharma for patients worldwide Nicole.
Pharmaceutical Technology
NOVEMBER 24, 2023
Vertex Pharmaceuticals has obtained approval from EC) for the label expansion of Kaftrio plus ivacaftor to treat children with CF.

Pharmacy Times
NOVEMBER 24, 2023
For pharmacists and pharmacy technicians, who work diligently to ensure our health and well-being, choosing the perfect holiday gift can be a thoughtful way to express gratitude and brighten their season.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Pharmaceutical Technology
NOVEMBER 24, 2023
The Sosei Heptares oral IBD drug was first licensed to GSK in 2020 and is currently in Phase I clinical development.

Pharmacy Times
NOVEMBER 24, 2023
In part 2 of this series, trauma-informed pharmacist Helen Sairany, PharmD, MBA, discusses the physical manifestations of burnout.
Pharmaceutical Technology
NOVEMBER 24, 2023
Access and buy GlobalData’s databook on the latest filings and grants activity for Johnson & Johnson here.

PharmaTech
NOVEMBER 24, 2023
Looking at the role that RSV and pediatric medical practices play in drug shortages.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Pharmaceutical Technology
NOVEMBER 24, 2023
Access and buy GlobalData’s databook on the latest filings and grants activity for F. Hoffmann-La Roche here.
Pharmaceutical Technology
NOVEMBER 24, 2023
Access and buy GlobalData’s databook on the latest filings and grants activity for Bristol-Myers Squibb here.

Pharmaceutical Technology
NOVEMBER 24, 2023
With more approved drugs coming through than ever before, the need to avoid confusing names to ensure patient safety is important.

Pharmaceutical Technology
NOVEMBER 24, 2023
The expansion comes as Novo is trying to maintain strong growth in T2D after the launch of Eli Lilly’s Zepbound this month.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
Pharmaceutical Technology
NOVEMBER 24, 2023
Access and buy GlobalData’s databook on the latest filings and grants activity for Novartis here.

Pharmaceutical Technology
NOVEMBER 24, 2023
China’s NMPA has approved ArkBio IND application for its respiratory syncytial virus (RSV) neutralising antibody, AK0610.
Pharmaceutical Technology
NOVEMBER 24, 2023
Ellipses Pharma has secured ODD from the US FDA for its next generation EP0031/A400, to potentially treat solid tumours.
Pharmaceutical Technology
NOVEMBER 24, 2023
Access and buy GlobalData’s databook on the latest filings and grants activity for Amgen here.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
Pharmaceutical Technology
NOVEMBER 24, 2023
Lupin has introduced the fixed-dose triple combination drug (FDC), Vilfuro-G, to manage chronic COPD in India.
Let's personalize your content