Biotech Startup Triveni Unveils $92M to Address Barrier to Treating Inflammation
MedCity News
NOVEMBER 5, 2023
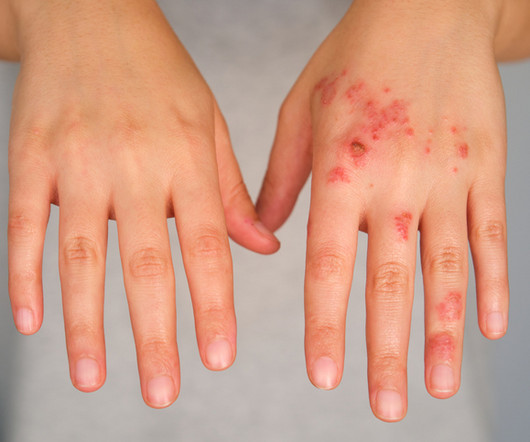
Available atopic dermatitis drugs work by blocking inflammation, but Triveni Bio is developing an antibody that addresses a root cause of the chronic condition. The startup is out of stealth to advance its lead program and a pipeline of other inflammation and immunology drugs.



































Let's personalize your content