Digital health tech: a solution to substance use disorders?
Pharmaceutical Technology
JULY 4, 2023
Substance use disorders leave people with long-term negative mental and physical health implications and can lead to death.

Pharmaceutical Technology
JULY 4, 2023
Substance use disorders leave people with long-term negative mental and physical health implications and can lead to death.

MedCity News
JULY 4, 2023
A new cohort of startups has graduated from PharmStars, a digital health accelerator. This latest group focused on women’s health and health equity.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
JULY 4, 2023
While drug shortages have been a major cause for concern over the past few years, medications to treat cancer are in particularly short supply.
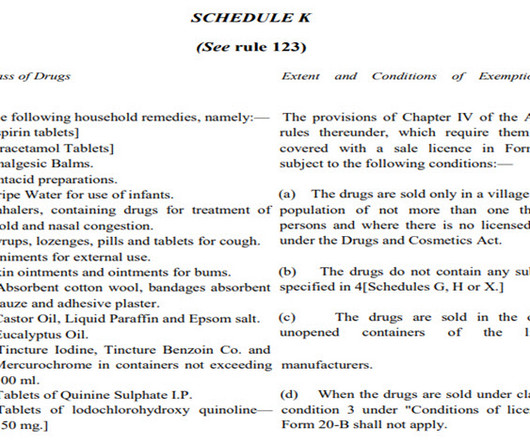
Pharmatutor
JULY 4, 2023
Reality Test of Medicine Shop without Registered Pharmacist admin Tue, 07/04/2023 - 15:39 ABOUT AUTHOR Dr. R. S. Thakur Renowned Professor of Pharmaceutical Fraternity & Former Member of Pharmacy Council of India. Email : drramsthakur@gmail.
European Pharmaceutical Review
JULY 4, 2023
The International Organization for Standardization (ISO) has published its new standard Sterilization of health care products — Microbiological methods — Part 3 Bacterial endotoxin testing ( ISO 11737-3:2023). The document contains requirements and guidance for testing for bacterial endotoxins. This includes products that must be non-pyrogenic based on either intended use or non-pyrogenic label claim, or both.
Pharmaceutical Technology
JULY 4, 2023
Abeona Therapeutics has raised $25m from its current select investors to launch and commercialise its cell therapy, EB-101.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

PharmaTimes
JULY 4, 2023
Therapy has been developed to reduce the frequency, duration and severity of migraine attacks - News - PharmaTimes

European Pharmaceutical Review
JULY 4, 2023
As drug development professionals know, global health crises like the COVID-19 pandemic provide the public with valuable insights into how clinical research and regulatory processes work. However, critical disciplines within the field responsible for ensuring safe and effective drug products, including quality assurance and compliance, remain mysterious or invisible to many.

PharmaTimes
JULY 4, 2023
Collaboration aims to revitalise two empty sites on the South Bank of London by providing several laboratories - News - PharmaTimes

European Pharmaceutical Review
JULY 4, 2023
Trends in capsule formulation In this Q&A, Recipharm’s Torkel Gren discusses developments in capsule formulation, including the shift away from gelatine and the potential for growth in the inhalation capsule market. Titanium dioxide: are there alternatives? Mike Tobyn from Bristol Myers Squibb, Jonathan Kaye from GSK, David Harris from MSD and Eli Lilly’s Jason Melnick discuss the role of E171 (titanium dioxide) in the identification of solid oral dosage forms.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

PharmaTech
JULY 4, 2023
Any responsible business or industry takes quality seriously. It’s crucial to customer safety and satisfaction, and its prioritization in the development of healthcare products and services – especially pharmaceuticals – is obviously even more so. Nonetheless, it can be easy in development for smaller teams and companies to overlook some components of Quality, despite governmental requirements around complex quality initiatives.
European Pharmaceutical Review
JULY 4, 2023
The rise in antimicrobial resistance , 1 lack of significant antimicrobial discovery in recent years, and increasing instances of multidrug-resistant (MDR) microorganisms 2 have propelled the interest in bacteriophage (Phage) therapy as a potential new course of treatment. Patients with implantable devices are more prone to biofilm-mediated infections, 3 while other infections such as skin structure infections, chronic lung diseases resulting from respiratory infections, and urinary tract infect

Evolve Your Success
JULY 4, 2023
What is the best way for a medical sales rep to approach a medical professional in a hospital setting? What is the state of nursing today, and what challenges is the profession taking? What do nurses need to know if they want to consider medical sales as an alternative career? These are three very interesting questions that our guest for this episode tackles.
European Pharmaceutical Review
JULY 4, 2023
JNJ-2113, the first and only oral interleukin-23 receptor (IL-23R) antagonist peptide in development for moderate-to-severe plaque psoriasis (PsO) has demonstrated positive results in a Phase II trial. Janssen’s Phase IIb FRONTIER 1 clinical trial for adult participants achieved all primary and secondary efficacy endpoints, according to topline results showcased by Bissonnette R, Pinter A, Ferris L, et al. at the World Congress of Dermatology 2023.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Pharmaceutical Technology
JULY 4, 2023
Amneal follows a successful year of approvals with six more approvals for generics across multiple indications.

PharmaTech
JULY 4, 2023
When evaluating a drug’s risk assessment for elemental impurities, one must consider all aspects of its lifecycle.

Pharmaceutical Technology
JULY 4, 2023
The US FDA has issued a complete response letter to Amneal Pharmaceuticals, declining to approve its IPX203 to treat Parkinson’s disease.

PharmaTech
JULY 4, 2023
When evaluating a drug’s risk assessment for elemental impurities, one must consider all aspects of its lifecycle.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
Pharmaceutical Technology
JULY 4, 2023
Moderna has filed a regulatory application seeking EMA approval for its modified Covid-19 vaccine targeting the XBB.1.5 sub-variant.

Pharmaceutical Technology
JULY 4, 2023
How can you reduce drop-out, save time and resources, and maintain participant engagement throughout the entirety of your trial?
Pharmaceutical Technology
JULY 4, 2023
NorthX Biologics has acquired Valneva’s clinical trial manufacturing unit in Stockholm, Sweden to bolster its expertise.
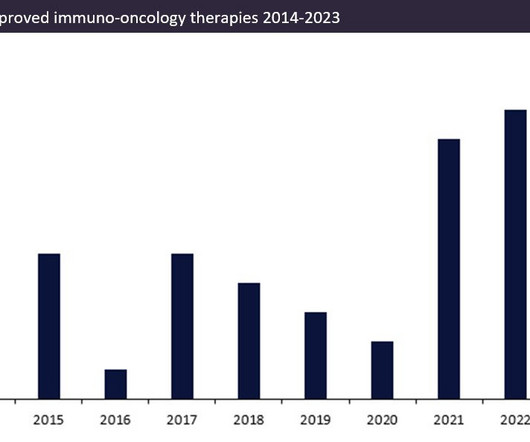
Pharmaceutical Technology
JULY 4, 2023
Over the last ten years, the number of approved innovator immuno-oncology therapies (immunotherapies) has increased.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
Pharmaceutical Technology
JULY 4, 2023
The venous thromboembolism (VTE) market is expected to grow from $3.6bn to $4.6bn globally, according to GlobalData.

Pharmaceutical Technology
JULY 4, 2023
Tonix Pharmaceuticals and Tonix Medicines have acquired two migraine products from Upsher-Smith Laboratories.

Pharmaceutical Technology
JULY 4, 2023
The spin-off will create a new global Phase I-IV CRO, patient access and technology solutions.

Pharmaceutical Technology
JULY 4, 2023
The gene therapy prevents neovascularisation from a single, intravitreally delivered injection.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic
Let's personalize your content