FDA approves bluebird's sickle cell disease gene therapy. Can Lyfgenia overcome CRISPR’s halo?
Fierce Pharma
DECEMBER 8, 2023
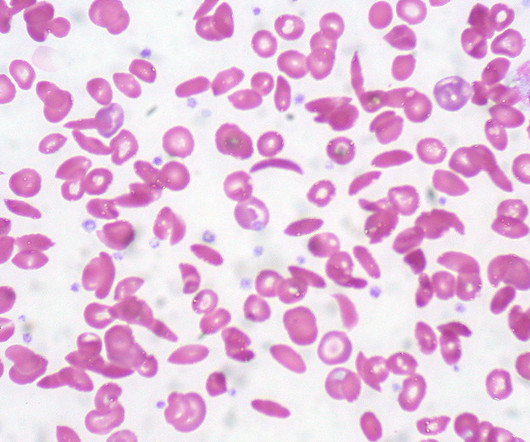
Alongside a historic approval for the first therapy utilizing the Nobel Prize-winning CRISPR/Cas9 gene-editing technology, the FDA has cleared a rival gene replacement therapy, also for sickle cell | Alongside a historic approval for the first therapy utilizing the Nobel Prize-winning CRISPR/Cas9 gene-editing technology, the FDA has cleared a rival gene replacement therapy, also for sickle cell disease (SCD).



































Let's personalize your content