Aldeyra's eye cancer hopes dashed as FDA issues rejection citing lack of clinical data
Fierce Pharma
JUNE 22, 2023
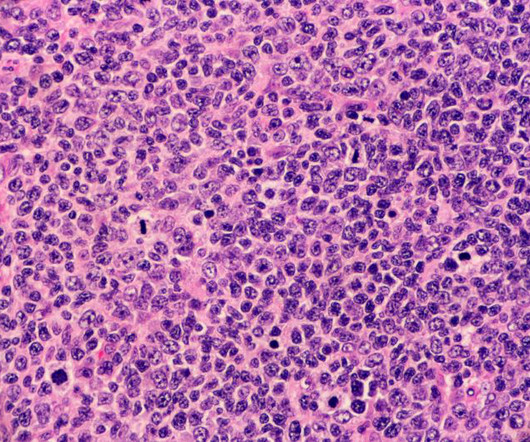
It's safe to say that a lack of clinical trials in a drug application raises red flags for the FDA. Aldeyra's bid for an approval in primary vitreoretinal lymphoma wasn't supported by clinical trial data as the company doesn't think a study in the rare and fatal disease is feasible, CEO Todd Brady, M.D.,



































Let's personalize your content