Novel oral chemotherapeutic holds potential for stomach cancer patients
European Pharmaceutical Review
JULY 5, 2022
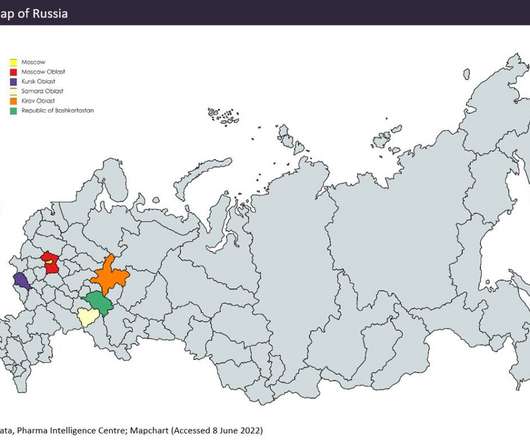
Several lifestyle factors have been shown to increase the risk of developing stomach cancer, including alcohol consumption, smoking and consuming foods preserved by salts. Oncoral (Ascelia Pharma) is a novel patented tablet formulation of irinotecan, currently in Phase II clinical development, for daily dosing at home.












Let's personalize your content