Cancer: Progress but a long way to go
World of DTC Marketing
MARCH 17, 2022
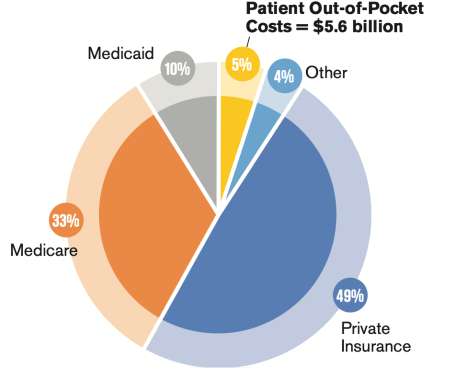
What treatment works in one patient may not work in another but we have to keep trying to beat cancer anyway we can. In recent years, early detection and treatment improvements have helped boost the 3-year survival rate for lung cancer from 21% in 2004 to 31% in 2015 through 2017. In 2018 cancer patients in the U.S.










Let's personalize your content