Biogen’s $900 million settlement signals scrutiny on speaker fees
Pharmaceutical Technology
OCTOBER 5, 2022
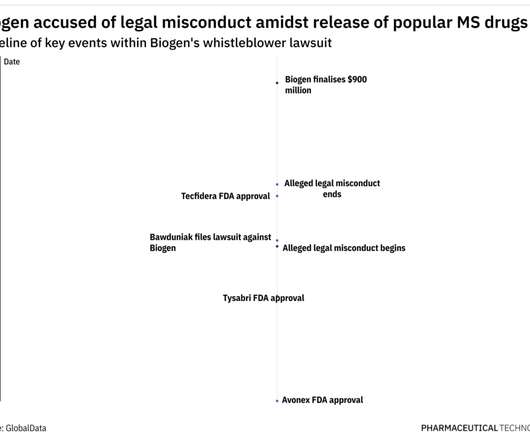
In 2009, former Biogen employee Michael Bawduniak filed a lawsuit claiming that Biogen had violated the False Claims Act and the Anti-Kickback Statute by providing millions of dollars to healthcare providers (HCPs) as an incentive to prescribe three of its multiple sclerosis (MS) drugs.










Let's personalize your content