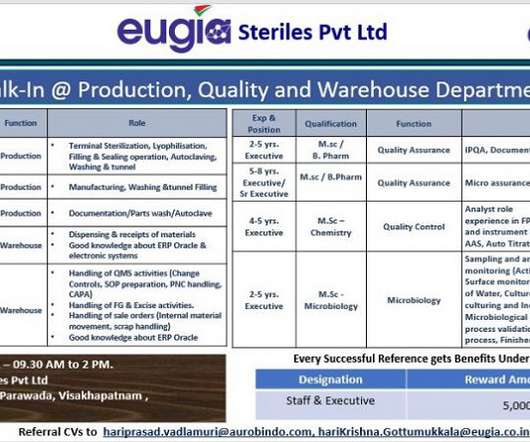
Eugia Pharma -Walk-In Interviews for Production/ Warehouse / Quality Assurance/ Quality Control/ Microbiology On 24th Sept’ 2022
Pharma Pathway
SEPTEMBER 20, 2022
Eugia Pharma Specialities Limited is Subsidiary of Aurobindo Pharma Limited, Aurobindo Pharma Ltd’ (APL). APL is a growing India multinational pharmaceutical manufacturing firm with turnover of over US$2.8 Job Description. Email Id: hariprasad.vadlamuri@aurobindo.com / harikrishna.gottumukkala@eugia.co.in.










Let's personalize your content