Celltrion’s Yuflyma receives US FDA approval for multiple conditions
Pharmaceutical Technology
MAY 25, 2023
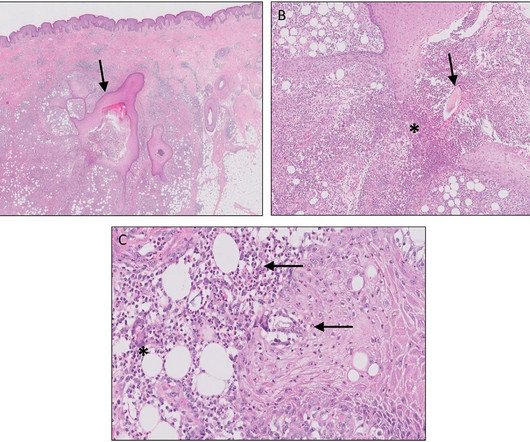
Celltrion USA has received approval from the US Food and Drug Administration (FDA) for Humira (adalimumab) biosimilar, Yuflyma (adalimumab-aaty) , for multiple indications. It will be offered to patients in prefilled syringe and autoinjector administration options.




















Let's personalize your content