Accord BioPharma’s HERCESSI secures FDA approval
Pharmaceutical Technology
APRIL 30, 2024
Accord BioPharma, the division of Intas Pharmaceuticals, has secured approval from the US FDA for HERCESSI, a biosimilar to Herceptin.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Pharmaceutical Technology
APRIL 30, 2024
Accord BioPharma, the division of Intas Pharmaceuticals, has secured approval from the US FDA for HERCESSI, a biosimilar to Herceptin.

Pharmaceutical Technology
OCTOBER 4, 2022
Pfizer has acquired commercial-stage biopharmaceutical firm Biohaven Pharmaceutical for $148.50 A CGRP receptor antagonist, NURTEC ODT is approved by the US Food and Drug Administration (FDA) for use in adults for acute treatment of migraine irrespective of aura status as well as for preventive episodic migraine treatment.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

MedCity News
APRIL 29, 2024
The FDA approved X4 Pharmaceuticals drug Xolremdi for treating WHIM syndrome. The post Drug Licensed from Sanofi Becomes First FDA-Approved Therapy for Ultra-Rare Primary Immunodeficiency appeared first on MedCity News.
MedCity News
MARCH 14, 2024
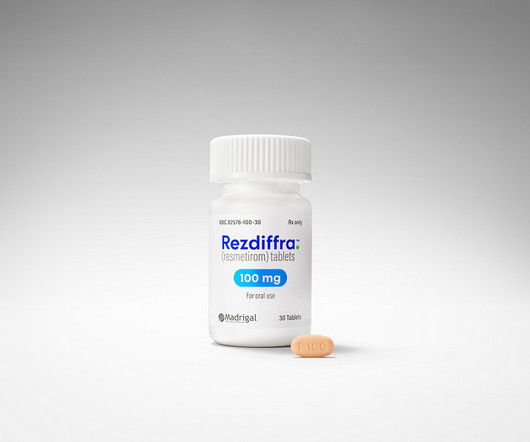
The FDA has approved Madrigal Pharmaceuticals drug Rezdiffra as a treatment for the fatty liver disease NASH (also called MASH). It’s the first treatment for the chronic condition, which has been growing in prevalence.

MedCity News
APRIL 1, 2024
The Otsuka Pharmaceutical and Click Therapeutics mobile app Rejoyn received FDA clearance for use as an adjunct to medication in the treatment of major depressive disorder. The post FDA Clears the First Digital Therapeutic for Depression, But Will Payers Cover It?

European Pharmaceutical Review
AUGUST 21, 2023
Q2 2023 biopharma market capitalisation results Eli Lilly “Eli Lilly witnessed the largest market capitalisation growth of 36.1 Lilly completes biopharma acquisitions GlobalData highlighted that Eli Lilly reported its synthetic peptide Mounjaro had $980 million in global sales in Q2. trillion in the Q1 of 2023 to $3.56

MedCity News
JANUARY 16, 2024
The FDA approved Vertex Pharmaceuticals’ gene therapy Casgevy for treating beta thalassemia, an inherited blood disease that leads to low levels of functioning hemoglobin. Last month, the one-time treatment won its first FDA nod for treating sickle cell disease.

MedCity News
FEBRUARY 12, 2024
FDA approval of Takeda Pharmaceutical drug Eohilia introduces a new therapeutic option for patients with eosinophilic esophagitis, inflammation of the esophagus that causes swallowing difficulty. It will compete against Dupixent, a Sanofi and Regeneron Pharmaceuticals drug already approved for this disorder.
Pharmaceutical Technology
OCTOBER 31, 2023
ArriVent Biopharma has received the US FDA's breakthrough therapy designation for furmonertinib to treat non-small cell lung cancer.
MedCity News
FEBRUARY 14, 2024
Ipsen’s Onivyde is now FDA approved as a first-line treatment for metastatic pancreatic cancer, triggering a milestone payment to Merrimack Pharmaceuticals, the drug’s original developer. Merrimack plans to dissolve operations but its shareholders will receive payouts from the Ipsen payment.

MedCity News
SEPTEMBER 20, 2023
ARS Pharmaceuticals frames its intranasal epinephrine spray as a needle-free alternative to products such as EpiPen. Though this spray won the backing of an FDA advisory committee, the agency is now requiring that ARS Pharma run another study to support a regulatory submission.

European Pharmaceutical Review
DECEMBER 6, 2022
GlobalData’s Pharmaceutical Intelligence Center Companies Database found that 16 of the 20 top biopharmaceutical companies indicated a decline in quarter-on-quarter (QoQ) market capitalisation. Cartic continued: “Regeneron Pharmaceuticals and Daiichi Sankyo witnessed the biggest growth in market capitalisation over Q3 2022 with 15.6

Pharmaceutical Technology
AUGUST 5, 2022
An orally administered selective inhibitor of complement component 5a receptor, Tavneos received approval from the US Food and Drug Administration (FDA) in October last year. The post Amgen to acquire biopharma firm ChemoCentryx for $3.7bn appeared first on Pharmaceutical Technology.

MedCity News
NOVEMBER 12, 2023
Takeda Pharmaceutical enzyme replacement drug Adzynma is the first FDA-approved therapy for a rare blood disorder affecting fewer than 1,000 Americans. Our regulatory recap also includes the much-anticipated approval of an Eli Lilly weight loss drug and a crackdown on what the FTC says are improperly listed pharma product patents.
MedCity News
MAY 11, 2023
The FDA decision makes the Otsuka Pharmaceutical and Lundbeck product the first drug approved for this indication. Rexulti, a drug for schizophrenia and depression, now has an additional approval for treating agitation in Alzheimer’s disease patients.

MedCity News
OCTOBER 13, 2023
Pfizer’s new FDA-approved ulcerative colitis drug Velsipity comes from its $6.7 billion Arena Pharmaceuticals acquisition. The small molecule will compete against blockbuster Bristol Myers Squibb drug Zeposia, which addresses the same target.

MedCity News
JULY 28, 2023
Biogen’s Reata Pharmaceuticals acquisition brings Skyclarys, the first and only FDA-approved therapy for the rare neuromuscular disease Friedreich’s ataxia. Biogen says Skyclarys complements the other neuromuscular drugs in its portfolio.

MedCity News
APRIL 1, 2024
The Otsuka Pharmaceutical and Click Therapeutics mobile app Rejoyn received FDA clearance for use as an adjunct to medication in the treatment of major depressive disorder. But Otsuka must overcome hurdles facing digital therapeutics products, which have yet to gain traction among payers.

Pharmaceutical Commerce
MAY 17, 2024
Business strategies and top news in the biotech / biopharma industry, including market access, supply chain distribution and more.
MedCity News
JUNE 23, 2023
The FDA again rejected Intercept Pharmaceuticals’ application seeking accelerated approval for its NASH drug and asked for more data. Instead, the biotech will stop all work in that fatty liver disease and focus on drugs for other serious but rare liver conditions.
MedCity News
OCTOBER 9, 2023
Despite an affirmative FDA advisory committee vote, the agency declined to approve Alnylam Pharmaceuticals’ Onpattro for treating the heart complications caused by a rare, inherited protein disorder. But Alnylam has other drugs candidates for the disease, including one expected to post Phase 3 data in the first half of 2024.
MedCity News
JUNE 29, 2023
The FDA approved Roctavian for treating hemophilia A. The regulatory decision makes the BioMarin Pharmaceutical product the first gene therapy for this inherited bleeding disorder.
European Pharmaceutical Review
NOVEMBER 23, 2022
The US Food and Drug Administration (FDA) has approved Hemgenix (etranacogene dezaparvovec), the first gene therapy for adults with Haemophilia B (congenital Factor IX deficiency) who currently use Factor IX prophylaxis therapy, or have current or historical life-threatening haemorrhage, or have repeated, serious spontaneous bleeding episodes.

MedCity News
MARCH 7, 2023
BioMarin Pharmaceutical’s submission of additional data for patients treated with its hemophilia A gene therapy, Roctavian, mean that the FDA will push out a regulatory decision to early summer. But it could be worth the wait as analysts anticipate FDA approval and project Roctavian becoming one of BioMarin’s biggest products.

MedCity News
MAY 30, 2023
Lexicon Pharmaceuticals drug Inpefa is now FDA-approved for heart failure. Inpefa is third in its class, but the biotech says its pill can reach a specific subset of patients, enabling it to stand apart from rival medicines from AstraZeneca and partners Eli Lilly and Boehringer Ingelheim.
MedCity News
DECEMBER 19, 2022
A Ferring Pharmaceuticals gene therapy is now FDA approved for treating bladder cancer that does not respond to an immunotherapy used to treat the cancer in its early stages. The Ferring gene therapy, Adstiladrin, turns bladder wall cells into tiny factories churning out a cancer-fighting therapeutic protein.

MedCity News
AUGUST 7, 2022
Acadia Pharmaceuticals drug Nuplazid failed to win FDA approval for the treatment of psychosis in Alzheimer’s disease patients. The regulator said that the data submitted were not from an adequate and well-controlled study and the company must run another clinical trial.
Pharmaceutical Technology
APRIL 6, 2023
The US Food and Drug Administration (FDA) has accepted Accord BioPharma’s Biologics Licence Application (BLA) for HLX02 (a proposed trastuzumab biosimilar) to treat HER2 cancer types. Accord BioPharma is the US specialty division of Intas Pharmaceuticals.

MedCity News
SEPTEMBER 25, 2022
Spectrum Pharmaceuticals and Oncopeptides each received separate negative advisory committee votes for their respective cancer drugs. In other recent regulatory news, clinical trial holds were placed and lifted and the FDA is seeking public comment on its draft guidance for including children in clinical trials.

MedCity News
JULY 1, 2022
Novartis is acquiring an FDA priority review voucher from Mallinckrodt Pharmaceuticals. The $100 million price tag is in the neighborhood of the going rate for these vouchers, which grant a company a shorter regulatory review timeline for a drug that addresses a rare or neglected disease.

MedCity News
JUNE 21, 2022
The planned FDA submission follows the report from AstraZeneca and Ionis Pharmaceuticals that their partnered drug, eplontersen, met the main goals of a pivotal study in treating nerve pain caused by hereditary transthyretin-mediated amyloidosis.
European Pharmaceutical Review
MAY 12, 2023
Swedish Orphan Biovitrum AB (Sobi ® ) has agreed to acquire CTI BioPharma for $1.7 The biopharma companies anticipate the transaction will help to change the treatment landscape for patients with rare diseases through new, innovative and effective medicines and therapies. The post $1.7b

MedCity News
SEPTEMBER 30, 2022
An Amylyx Pharmaceuticals drug developed to slow the progression of amyotrophic lateral sclerosis is now approved, making it just the third FDA-approved treatment for the disease. Amylyx will market its new product under the name “Relyvrio.”.
MedCity News
NOVEMBER 28, 2022
In rejecting Spectrum Pharmaceuticals drug poziotinib, the FDA said the biotech needs to generate more data from another clinical trial. Instead, Spectrum is turning the company’s focus to commercializing its recently approved product for treating a common cancer complication.
Pharmaceutical Technology
FEBRUARY 16, 2023
The US Food and Drug Administration (FDA) has accepted the biologics licence application (BLA) for Shanghai Henlius Biotech ’s proposed biosimilar HLX02 (trastuzumab for injection). Accord BioPharma (Accord US), the business partner of Shanghai Henlius Biotech, has submitted the BLA.

MedCity News
JANUARY 22, 2024
In its Phase 3 test, Ionis Pharmaceuticals drug donidalorsen reduced the frequency of swelling attacks caused by the rare disease hereditary angioedema. If Ionis can commercialize this drug, competition would include products from Takeda Pharmaceutical and BioCryst Pharmaceuticals.
Pharmaceutical Technology
FEBRUARY 15, 2023
Kinnate Biopharma has received Fast Track designation from the US Food and Drug Administration (FDA) for its pan-FGFR inhibitor, KIN-3248, to treat unresectable, locally advanced or metastatic cholangiocarcinoma (CCA). Kinnate Biopharma expects to receive initial dose escalation data in the second half of this year.

MedCity News
MAY 8, 2024
Otsuka Pharmaceutical is launching a subsidiary that will commercialize its digital therapeutic Rejoyn — as well as other digital therapeutics and connected health products down the line. Last month, Rejoyn became the first FDA-cleared digital therapeutic for patients with depression.
MedCity News
AUGUST 3, 2022
Alnylam Pharmaceuticals drug Onpattro, an FDA-approved treatment for nerve pain caused by hereditary transthyretin amyloidosis, now has Phase 3 data showing it can also help the much larger group of patients suffering heart problems from the rare protein disease.
Pharmaceutical Technology
DECEMBER 20, 2022
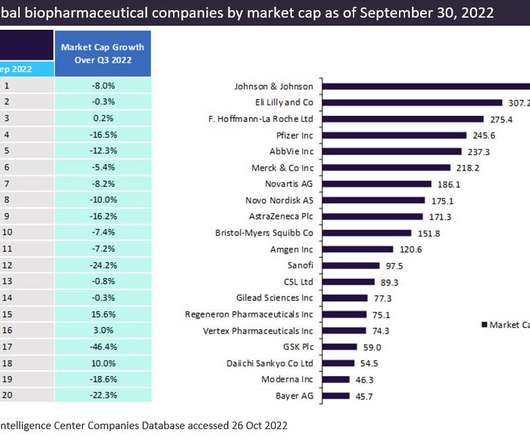
Eli Lilly saw strong global drug sales of $187m in Q3 for Mounjaro (tirzepatide), its new type 2 diabetes therapy, which was approved by the FDA in May 2022, according to GlobalData’s Drugs Database Pharma Intelligence Center. and 10%, respectively. GSK and Sanofi’s share prices dropped sharply by 46.4%

MedCity News
MARCH 6, 2024
This case, about the claim that Merck failed to warn patients about the risk of typical femoral fracture, reaches past this suit to potentially change pharmaceutical companies’ relationship with the FDA.

MedCity News
MARCH 14, 2024
Takeda Pharmaceutical markets the only FDA-approved treatment for this rare disease, but the Japanese pharmaceutical giant plans to stop making the drug. AstraZeneca is acquiring Amolyt Pharma to get eneboparatide, a drug in Phase 3 development for treating hypoparathyroidism.
MedCity News
DECEMBER 8, 2023
The first gene therapies for sickle cell disease are now approved: Casgevy from Vertex Pharmaceuticals Casgevy and Lyfgenia from Bluebird Bio. Despite the same-day approval, key differences give one of these therapies commercialization advantages over the other.
MedCity News
MARCH 12, 2024
With pimavanserin’s Phase 3 failure in schizophrenia, Acadia Pharmaceuticals said it will no longer run clinical trials for the drug, whose lone approval is for treating psychosis from Parkinson’s disease. The FDA previously rebuffed regulatory submissions for the drug in dementia and Alzheimer’s disease.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content