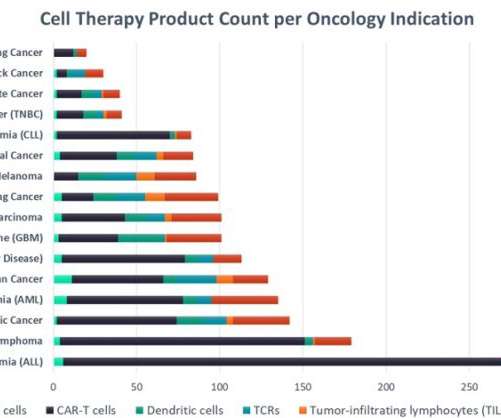
Acute lymphocytic leukaemia leads in cell therapy clinical development
Pharmaceutical Technology
JULY 12, 2022
The success of chimeric antigen receptor T-cells (CAR-T) in blood cancers has led to the US Food and Drug Administration's (FDA) approval of six products with a pipeline of cell therapies that numbers in the thousands. The total market for cell therapies in oncology is projected to exceed $37bn worldwide by 2028.














Let's personalize your content