Astellas facing generics to big-selling Lexiscan product in US
pharmaphorum
SEPTEMBER 26, 2022
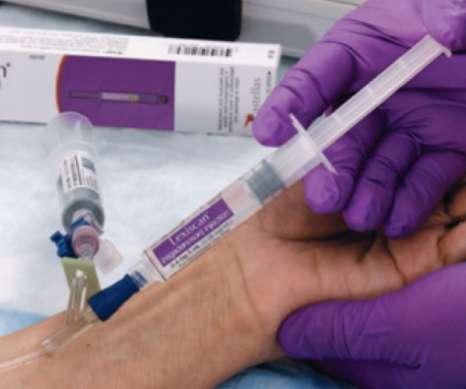
Astellas has won a reprieve in its attempt to stop Pfizer’s generic medicines unit Hospira launching a copycat version of its pharmacologic stress agent Lexiscan in the US – but only for a couple of weeks. AmphaStar and Glenmark Pharma also claimed FDA approval for versions of the drug earlier this year.











Let's personalize your content