A broad range of unmet needs remains in the immuno-oncology space
Pharmaceutical Technology
MAY 2, 2023
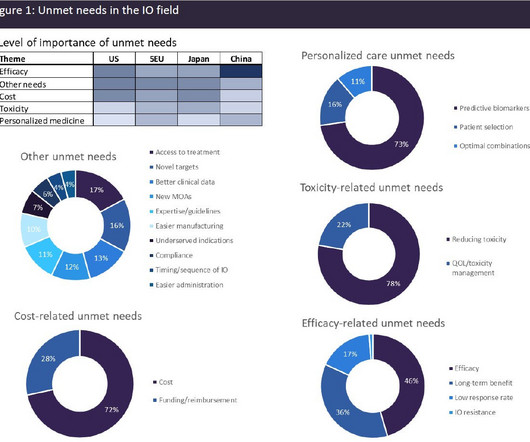
In contrast to ICIs, which are utilised against solid tumours, cell therapies and bispecifics have been transformational in the haematological cancer settings, with approvals across a range of leukaemias and lymphomas. Cost-related unmet needs also scored highly.










Let's personalize your content