Upcoming sickle cell gene therapies cost effective at $2 million, says ICER
Pharmaceutical Technology
APRIL 13, 2023
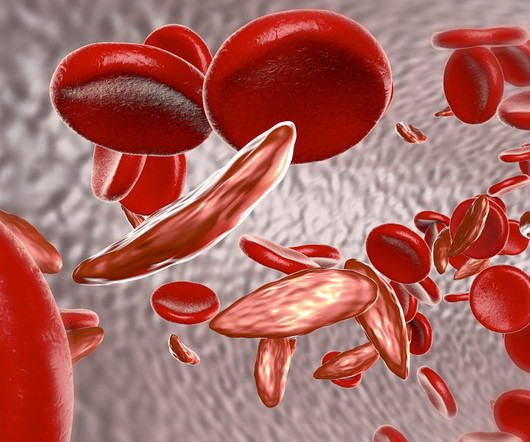
Two gene therapies up for approval this year for sickle cell disease could be cost effective in some cases at a $2 million price point, based on a draft evidence report published by the Institute for Clinical and Economic Review (ICER). Also known as lovo-cel, bluebird bio’s product is a lentiviral gene therapy.








Let's personalize your content