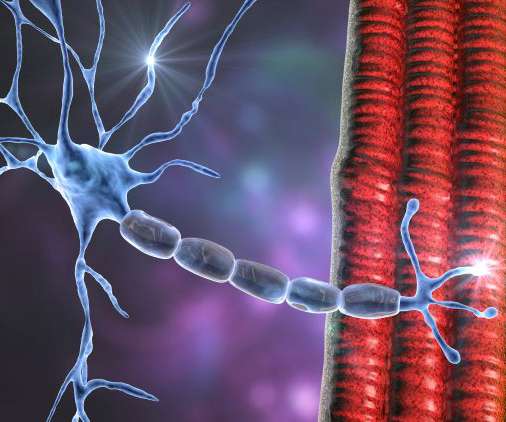
Converging knowledge and technology to transform neuromuscular disease treatment
European Pharmaceutical Review
DECEMBER 19, 2022
1 The resulting weakness can lead to twitching, cramps, pain, and joint and movement problems. 1 Types of NMD include muscular dystrophies, inflammatory myopathies and motor neurone diseases, such as spinal muscular atrophy (SMA) and amyotrophic lateral sclerosis (ALS) among others. Journal of Neuromuscular Diseases.












Let's personalize your content