Paragonix Technologies Receives FDA Clearance for BAROguard™ Donor Lung Preservation System
Legacy MEDSearch
AUGUST 25, 2023
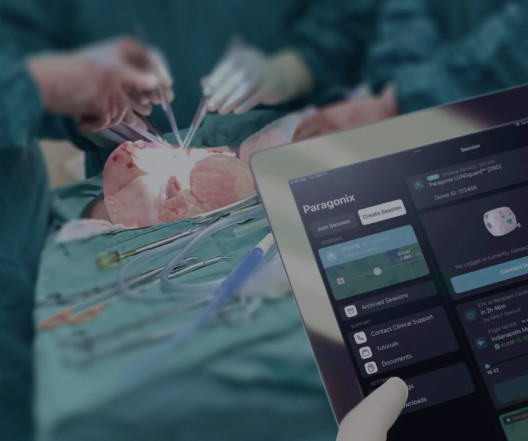
Paragonix Technologies , a leading organ transplant company, received US Food and Drug Administration (“FDA”) clearance for its next-generation donor lung preservation system, BAROguard. Under current clinical practice, donor lungs are preserved and transported in an inflated state from donor to recipient site.










Let's personalize your content