Lazurite is Recognized in the 2022 Medical Device Network Excellence Awards & Rankings for its ArthroFree™ System
Legacy MEDSearch
DECEMBER 7, 2022
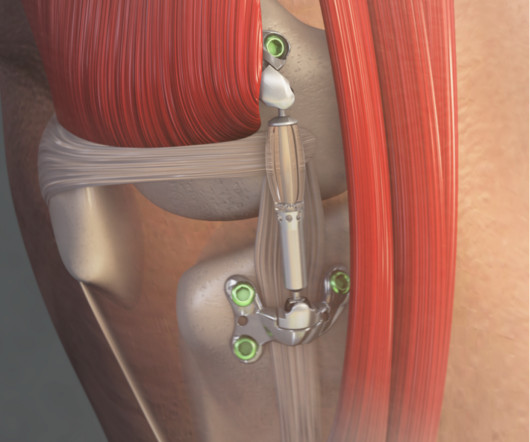
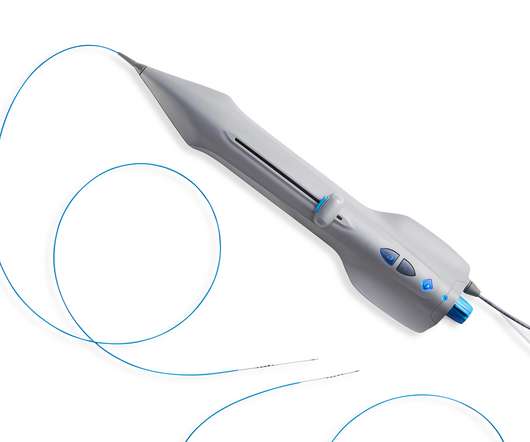
Food and Drug Administration market clearance for the ArthroFree System. This was followed by additional partnerships with Hospital for Special Surgery (HSS) in New York, University Hospitals of Cleveland, and LG Electronics USA.”. “We The company hit an important milestone in March 2022 when it received U.S.













Let's personalize your content