Burning Rock Received FDA Breakthrough Device Designation for its OverC™ Multi-Cancer Detection Blood Test
Legacy MEDSearch
JANUARY 3, 2023
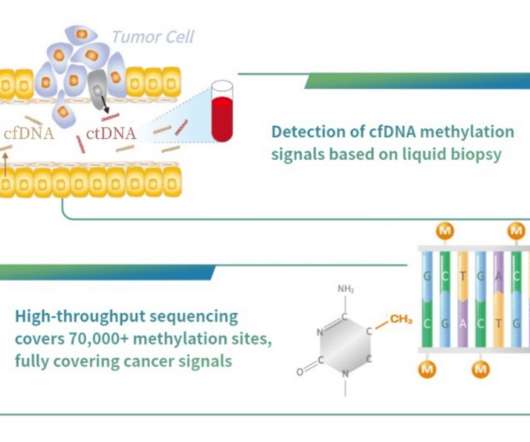
Burning Rock, a company focused on the application of next generation sequencing (NGS) technology in the field of precision oncology, announced that its OverC Multi-Cancer Detection Blood Test (MCDBT) has been granted Breakthrough Device Designation by the US Food and Drug Administration (FDA), which is the third of its kind globally.













Let's personalize your content