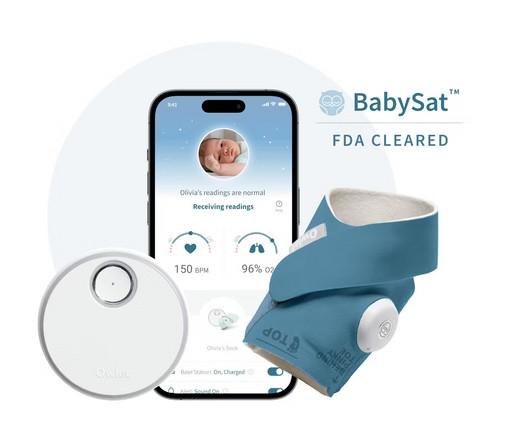
Owlet Announces FDA-Clearance of the First Prescription Pulse Oximetry Sock for Infants
Legacy MEDSearch
JUNE 20, 2023
Food and Drug Administration (“FDA”) of BabySat , the first medical pulse-oximetry device featuring its advanced, wire-free sock design. The post Owlet Announces FDA-Clearance of the First Prescription Pulse Oximetry Sock for Infants appeared first on Legacy MedSearch | Medical Device Recruiters.














Let's personalize your content