US FDA grants orphan drug status for XORTX’s oxypurinol
Pharmaceutical Technology
APRIL 24, 2023
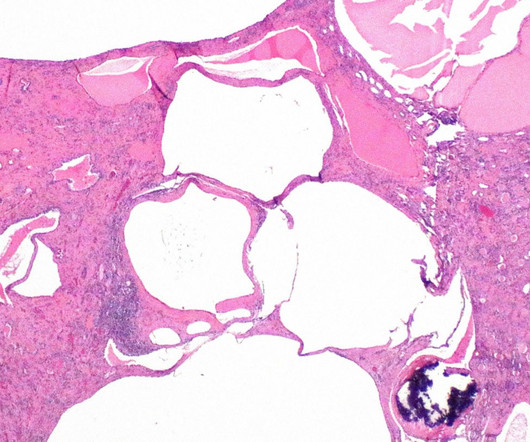
The US Food and Drug Administration (FDA) has granted orphan drug designation (ODD) to XORTX Therapeutics’ oxypurinol to treat autosomal dominant polycystic kidney disease (ADPKD) patients. The company noted that the ODD from the FDA is not an approval for the use of XORLO, a formulation of oxypurinol.














Let's personalize your content