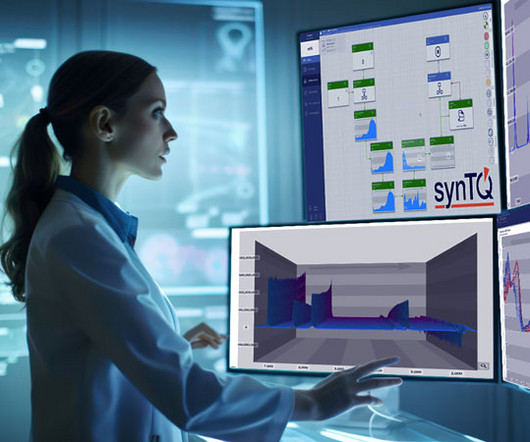
Elevating pharmaceutical manufacturing processes with real-time insights
European Pharmaceutical Review
DECEMBER 4, 2023
The (bio)pharmaceutical industry is currently facing numerous hurdles in its path to high-efficiency production that are more complex and pressing than ever before. Simultaneously, there is increasing need for stringent quality control and traceability throughout the supply chain to ensure patient safety. principles.














Let's personalize your content