Bluebird won't play off Vertex in deciding price for its lovo-cel gene therapy, exec says
Fierce Pharma
NOVEMBER 7, 2023
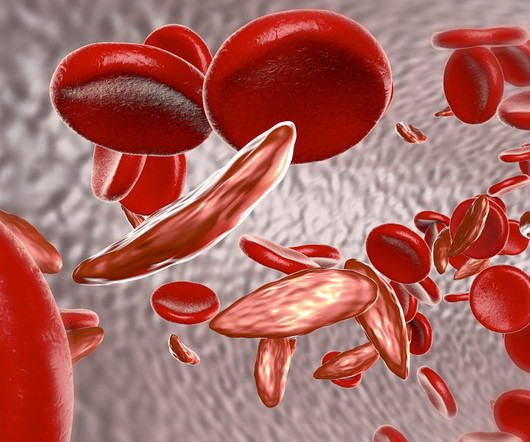
Two long-awaited treatments for sickle cell disease (SCD) are on the docket for FDA decisions next month. Two long-awaited treatments for sickle cell disease (SCD) are on the docket for FDA decisions next month. Vertex and CRISPR Therapeutics are up first with a Dec. |










Let's personalize your content