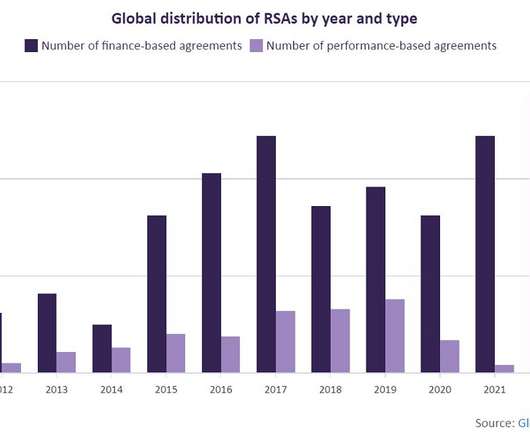
Risk-sharing agreements are growing at a rate of 24%
Pharmaceutical Technology
FEBRUARY 10, 2023
This is not the first treatment to come with a high price tag. For example, in Sweden, the proposed arrangement for Sanofi / Regeneron’s Dupixent (dupilumab) included a tripartite price negotiation involving Sanofi, the Dental and Pharmaceutical Benefits Agency (Tandvårds- och läkemedelsförmånsverket, TLV), and local councils.









Let's personalize your content