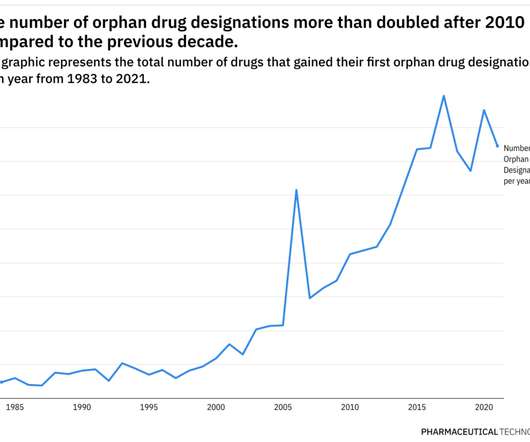
Rare Disease Spotlight – tracing the rise of orphan drug designations over almost 40 years
Pharmaceutical Technology
JUNE 29, 2022
This year has already been eventful when it comes to the development of therapies for rare diseases. Additionally, pricing and access for rare disease therapies continue to be scrutinized closely. Most, if not all, of these therapies used the FDA’s Orphan Drug Designation to aid their development plans.








Let's personalize your content